FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS)
Janssen COVID-19 Vaccine by
Drug Labeling and Warnings
Janssen COVID-19 Vaccine by is a Other medication manufactured, distributed, or labeled by Janssen Products, LP, Janssen, Jannsen Biologics B.V., Grand River Aseptic Manufacturing, Inc., Emergent Manufacturing Operations Baltimore LLC, Packaging Coordinators, LLC, Catalent Indiana, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
JANSSEN COVID-19 VACCINE- ad26.cov2.s injection, suspension
Janssen Products, LP
----------
FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS)
EMERGENCY USE AUTHORIZATION (EUA) OF THE JANSSEN COVID-19 VACCINE TO PREVENT CORONAVIRUS DISEASE 2019 (COVID-19)
WARNING: THROMBOSIS WITH THROMBOCYTOPENIA SYNDROME
See Full EUA Prescribing Information for complete warning.
The Janssen COVID-19 Vaccine can cause thrombosis with thrombocytopenia syndrome (TTS) which may be life-threatening.
TTS may involve thrombosis at unusual locations for a thrombus (i.e., cerebral vein, visceral artery or vein, extremity artery, central artery or vein) or in an extremity vein or pulmonary artery.
Among reported cases of TTS following administration of the Janssen COVID-19 Vaccine, symptoms began approximately one to two weeks after vaccination.
Instruct Janssen COVID-19 Vaccine recipients to seek immediate medical attention for shortness of breath, chest pain, leg swelling, persistent abdominal pain, neurological symptoms (including severe or persistent headaches or blurred vision), or petechiae beyond the site of vaccination.
The clinical course of TTS following administration of the Janssen COVID-19 Vaccine shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS, the use of heparin may be harmful and alternative treatments may be needed.
Do not administer the Janssen COVID-19 Vaccine to individuals with a history of thrombosis with thrombocytopenia following the Janssen COVID-19 Vaccine or any other adenovirus-vectored COVID-19 vaccine.
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) to permit the emergency use of the unapproved product, the Janssen COVID-19 Vaccine, for active immunization to prevent COVID-19 in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
SUMMARY OF INSTRUCTIONS FOR COVID-19 VACCINATION PROVIDERS
Vaccination providers enrolled in the federal COVID-19 Vaccination Program must report all vaccine administration errors, all serious adverse events, cases of myocarditis, cases of pericarditis, cases of Multisystem Inflammatory Syndrome (MIS) in adults, and cases of COVID-19 that result in hospitalization or death following administration of the Janssen COVID-19 Vaccine. See " MANDATORY REQUIREMENTS FOR THE JANSSEN COVID-19 VACCINE ADMINISTRATION UNDER EMERGENCY USE AUTHORIZATION" for reporting requirements.
The Janssen COVID-19 vaccine is authorized for use under an EUA for active immunization to prevent COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
The Janssen COVID-19 Vaccine is a suspension for intramuscular injection.
Primary Vaccination
The primary vaccination regimen for the Janssen COVID-19 Vaccine is a single-dose (0.5 mL).
Booster Dose
A first booster dose (0.5 mL) of Janssen COVID-19 Vaccine may be administered at least 2 months after completion of primary vaccination with an authorized or approved COVID-19 vaccine.
See this Fact Sheet for instructions for preparation and administration. This Fact Sheet may have been updated. For the most recent Fact Sheet, please see www.janssencovid19vaccine.com.
For information on clinical trials that are testing the use of the Janssen COVID-19 Vaccine for active immunization against COVID-19, please see www.clinicaltrials.gov.
DESCRIPTION OF COVID-19
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the novel coronavirus, SARS-CoV-2, that appeared in late 2019. It is predominantly a respiratory illness that can affect other organs. People with COVID-19 have reported a wide range of symptoms, ranging from mild symptoms to severe illness. Symptoms may appear 2 to 14 days after exposure to the virus. Symptoms may include: fever or chills; cough; shortness of breath; fatigue; muscle or body aches; headache; new loss of taste or smell; sore throat; congestion or runny nose; nausea or vomiting; diarrhea.
DOSAGE AND ADMINISTRATION
The storage and handling information in this Fact Sheet supersedes the storage and handling information on the carton and vial labels.
Storage and Handling
Storage Prior to First Puncture of the Vaccine Vial
Store unpunctured multi-dose vials of the Janssen COVID-19 Vaccine at 2°C to 8°C (36°F to 46°F) and protect from light. Do not store frozen.
Unpunctured vials of Janssen COVID-19 Vaccine may be stored between 9°C to 25°C (47°F to 77°F) for up to 12 hours.
The Janssen COVID-19 Vaccine is initially stored frozen by the manufacturer, then shipped at 2°C to 8°C (36°F to 46°F). If vaccine is still frozen upon receipt, thaw at 2°C to 8°C (36°F to 46°F). If needed immediately, thaw at room temperature (maximally 25°C/77°F). At room temperature (maximally 25°C/77°F), a carton of 10 vials will take approximately 4 hours to thaw, and an individual vial will take approximately 1 hour to thaw. Do not refreeze once thawed.
Storage After First Puncture of the Vaccine Vial
After the first dose has been withdrawn, hold the vial between 2° to 8°C (36° to 46°F) for up to 6 hours or at room temperature (maximally 25°C/77°F) for up to 2 hours. Discard the vial if vaccine is not used within these times.
Dose and Schedule
The Janssen COVID-19 vaccine is authorized for use under an EUA for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
Primary Vaccination
The primary vaccination regimen for the Janssen COVID-19 Vaccine is a single-dose (0.5 mL).
Booster Dose
A first booster dose (0.5 mL) of Janssen COVID-19 Vaccine may be administered at least 2 months after completion of primary vaccination with an authorized or approved COVID-19 vaccine.
Dose Preparation
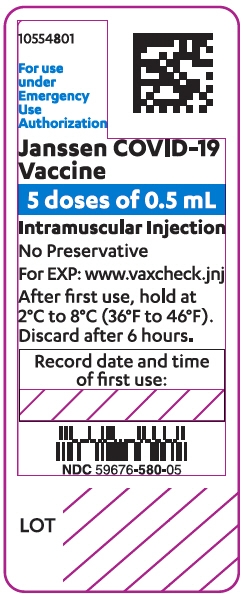
- The Janssen COVID-19 Vaccine is a colorless to slightly yellow, clear to very opalescent suspension. Visually inspect the Janssen COVID-19 Vaccine vials for particulate matter and discoloration prior to administration. If either of these conditions exists, do not administer the vaccine.
- Before withdrawing each dose of vaccine, carefully mix the contents of the multi-dose vial by swirling gently in an upright position for 10 seconds. Do not shake.
- Each dose is 0.5 mL. Each vial contains five doses. Do not pool excess vaccine from multiple vials.
- The Janssen COVID-19 Vaccine does not contain a preservative. Record the date and time of first use on the Janssen COVID-19 Vaccine vial label. After the first dose has been withdrawn, hold the vial between 2° to 8°C (36° to 46°F) for up to 6 hours or at room temperature (maximally 25°C/77°F) for up to 2 hours. Discard if vaccine is not used within these times.
Administration
Visually inspect each dose in the dosing syringe prior to administration. The Janssen COVID-19 Vaccine is a colorless to slightly yellow, clear to very opalescent suspension. During the visual inspection,
- verify the final dosing volume of 0.5 mL.
- confirm there are no particulates and that no discoloration is observed.
- do not administer if vaccine is discolored or contains particulate matter.
Administer the Janssen COVID-19 Vaccine intramuscularly.
CONTRAINDICATIONS
Severe Allergic Reactions
Do not administer the Janssen COVID-19 Vaccine to individuals with a known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the Janssen COVID-19 Vaccine (see Full EUA Prescribing Information) .
Thrombosis with Thrombocytopenia
Do not administer the Janssen COVID-19 Vaccine to individuals with a history of thrombosis with thrombocytopenia following the Janssen COVID-19 Vaccine or any other adenovirus-vectored COVID-19 vaccines (e.g., AstraZeneca's COVID-19 vaccine which is not authorized or approved in the United States) ( see Full EUA Prescribing Information).
WARNINGS
Management of Acute Allergic Reactions
Appropriate medical treatment to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of the Janssen COVID-19 Vaccine.
Monitor Janssen COVID-19 Vaccine recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention guidelines ( https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
Thrombosis with Thrombocytopenia Syndrome (TTS)
Reports to the Vaccine Adverse Events Reporting System (VAERS), a passive surveillance system, provide evidence for an increased risk of thrombosis with thrombocytopenia syndrome (TTS) with onset of symptoms approximately one to two weeks after administration of the Janssen COVID-19 Vaccine. An analysis of VAERS reports of TTS following the receipt of the Janssen COVID-19 Vaccine used the following case definition:
- a thrombosis in an unusual location for a thrombus (i.e., cerebral vein, visceral artery or vein, extremity artery, central artery or vein) and new-onset thrombocytopenia (i.e., platelet count <150,000/μL) occurring any time after vaccination;
or - new-onset thrombocytopenia (i.e., platelet count <150,000/μL), thrombosis in an extremity vein or pulmonary artery in the absence of thrombosis at an unusual location, and a positive anti-PF4 antibody ELISA test or functional Heparin-Induced Thrombocytopenia (HIT) platelet test occurring any time after vaccination.
Cases of TTS following administration of the Janssen COVID-19 Vaccine have been reported in males and females, in a wide age range of individuals 18 years and older, with the highest reporting rate (approximately 8 cases per 1,000,000 doses administered) in females ages 30–49 years; overall, approximately 15% of TTS cases have been fatal. Currently available evidence supports a causal relationship between TTS and the Janssen COVID-19 Vaccine. The clinical course of these events shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following administration of the Janssen COVID-19 Vaccine, the use of heparin may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended. The American Society of Hematology has published considerations relevant to the diagnosis and treatment of TTS following administration of the Janssen COVID-19 Vaccine ( https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia). ( see Full EUA Prescribing Information).
Immune Thrombocytopenia (ITP)
Reports of adverse events following use of the Janssen COVID-19 Vaccine under emergency use authorization suggest an increased risk of immune thrombocytopenia (ITP) during the 42 days following vaccination. Individuals with a history of ITP should discuss with their healthcare provider the risk of ITP and the potential need for platelet monitoring following vaccination with the Janssen COVID-19 Vaccine.
Guillain-Barré Syndrome
Reports of adverse events following use of the Janssen COVID-19 Vaccine under emergency use authorization suggest an increased risk of Guillain-Barré syndrome during the 42 days following vaccination.
Myocarditis and Pericarditis
Reports of adverse events following use of the Janssen COVID-19 Vaccine under emergency use authorization suggest increased risks of myocarditis and pericarditis, particularly within the period 0 through 7 days following vaccination.
The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis ( https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#myocarditis-pericarditis).
Altered Immunocompetence
Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to the Janssen COVID-19 Vaccine.
Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines. Procedures should be in place to avoid injury from fainting.
Limitations of Vaccine Effectiveness
The Janssen COVID-19 Vaccine may not protect all vaccinated individuals.
ADVERSE REACTIONS
Adverse Reactions in Clinical Trials
Adverse reactions reported in a clinical trial following administration of the Janssen COVID-19 Vaccine include injection site pain, headache, fatigue, myalgia, nausea, fever, injection site erythema and injection site swelling. In clinical studies, severe allergic reactions, including anaphylaxis, have been reported following administration of the Janssen COVID-19 Vaccine (see Full EUA Prescribing Information) .
Adverse Reactions Identified during Post Authorization Use
Anaphylaxis and other severe allergic reactions, thrombosis with thrombocytopenia, immune thrombocytopenia, Guillain-Barré syndrome, myocarditis, pericarditis, and capillary leak syndrome have been reported following administration of the Janssen COVID-19 Vaccine during mass vaccination outside of clinical trials (see Full EUA Prescribing Information) .
Additional adverse reactions, some of which may be serious, may become apparent with more widespread use of the Janssen COVID-19 Vaccine.
USE WITH OTHER VACCINES
There is no information on the co-administration of the Janssen COVID-19 Vaccine with other vaccines.
INFORMATION TO PROVIDE TO VACCINE RECIPIENTS/CAREGIVERS
As the vaccination provider, you must communicate to the recipient or their caregiver, information consistent with the "Fact Sheet for Recipients and Caregivers" (and provide a copy or direct the individual to the website www.janssencovid19vaccine.com to obtain the Fact Sheet) prior to the individual receiving the Janssen COVID-19 Vaccine, including:
- FDA has authorized the emergency use of the Janssen COVID-19 Vaccine, which is not an FDA approved vaccine.
- There is an option to accept or refuse the Janssen COVID-19 Vaccine.
- The significant known and potential risks and benefits of the Janssen COVID-19 Vaccine, and the extent to which such risks and benefits are unknown.
- Information about available alternative vaccines and the risks and benefits of those alternatives.
For information on clinical trials that are testing the use of the Janssen COVID-19 Vaccine to prevent COVID-19, please see www.clinicaltrials.gov.
Provide a vaccination card to the recipient or their caregiver with the name of the vaccine ("Janssen COVID-19 Vaccine") and date of administration to document vaccination.
Provide the v-safe information sheet to vaccine recipients/caregivers and encourage vaccine recipients to participate in v-safe. V-safe is a voluntary smartphone-based tool that uses text messaging and web surveys to check in with people who have been vaccinated to identify potential side effects after COVID-19 vaccination. V-safe asks questions that help CDC monitor the safety of COVID-19 vaccines. V-safe also provides live telephone follow-up by CDC if participants report a significant health impact following COVID-19 vaccination. For more information, visit: www.cdc.gov/vsafe.
MANDATORY REQUIREMENTS FOR JANSSEN COVID-19 VACCINE ADMINISTRATION UNDER EMERGENCY USE AUTHORIZATION
In order to mitigate the risks of using this unapproved product under EUA and to optimize the potential benefit of the Janssen COVID-19 Vaccine, the following items are required. Use of unapproved Janssen COVID-19 Vaccine for active immunization to prevent COVID-19 under this EUA is limited to the following (all requirements must be met):
- The Janssen COVID-19 Vaccine is authorized for use in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
- The vaccination provider must communicate to the individual receiving the Janssen COVID-19 Vaccine or their caregiver, information consistent with the "Fact Sheet for Recipients and Caregivers" prior to the individual receiving the Janssen COVID-19 Vaccine.
- The vaccination provider must include vaccination information in the state/local jurisdiction's Immunization Information System (IIS) or other designated system.
- The vaccination provider is responsible for mandatory reporting of the following to the Vaccine Adverse Event Reporting System (VAERS):
- vaccine administration errors whether or not associated with an adverse event,
- serious adverse events* (irrespective of attribution to vaccination),
- cases of myocarditis,
- cases of pericarditis,
- cases of Multisystem Inflammatory Syndrome (MIS) in adults, and
- cases of COVID-19 that result in hospitalization or death.
- The vaccination provider is responsible for responding to FDA requests for information about vaccine administration errors, adverse events, cases of myocarditis, cases of pericarditis, cases of MIS in adults, and cases of COVID-19 that result in hospitalization or death following administration of the Janssen COVID-19 Vaccine to recipients.
* Serious adverse events are defined as:
- Death;
- A life-threatening adverse event;
- Inpatient hospitalization or prolongation of existing hospitalization;
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions;
- A congenital anomaly/birth defect;
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent one of the outcomes listed above.
OTHER ADVERSE EVENT REPORTING TO VAERS AND JANSSEN BIOTECH, INC.
Vaccination providers may report to VAERS other adverse events that are not required to be reported using the contact information above.
To the extent feasible, report adverse events to Janssen Biotech, Inc. using the contact information below or by providing a copy of the VAERS form to Janssen Biotech, Inc:
| Fax number | Telephone numbers | |
|---|---|---|
| JNJvaccineAE@its.jnj.com | 215-293-9955 | US Toll Free: 1-800-565-4008
US Toll: (908) 455-9922 |
ADDITIONAL INFORMATION
For general questions or to access the most recent Janssen COVID-19 Vaccine Fact Sheets, scan the QR code using your device, visit www.janssencovid19vaccine.com or call the telephone numbers provided below.
| QR Code | Fact Sheets Website | Telephone numbers |
|---|---|---|
|
| www.janssencovid19vaccine.com. | US Toll Free: 1-800-565-4008
US Toll: 1-908-455-9922 |
AVAILABLE ALTERNATIVES
COMIRNATY (COVID-19 Vaccine, mRNA) and SPIKEVAX (COVID-19 Vaccine, mRNA) are FDA-approved vaccines to prevent COVID-19 caused by SARS-CoV-2. There may be clinical trials or availability under EUA of other COVID-19 vaccines.
FEDERAL COVID-19 VACCINATION PROGRAM
This vaccine is being made available for emergency use exclusively through the CDC COVID-19 Vaccination Program (the Vaccination Program). Healthcare providers must enroll as providers in the Vaccination Program and comply with the provider requirements. Vaccination providers may not charge any fee for the vaccine and may not charge the vaccine recipient any out-of-pocket charge for administration. However, vaccination providers may seek appropriate reimbursement from a program or plan that covers COVID-19 vaccine administration fees for the vaccine recipient (private insurance, Medicare, Medicaid, HRSA COVID-19 Uninsured Program for non-insured recipients). For information regarding provider requirements and enrollment in the CDC COVID-19 Vaccination Program, see https://www.cdc.gov/vaccines/covid-19/provider-enrollment.html.
Individuals becoming aware of any potential violations of the CDC COVID-19 Vaccination Program requirements are encouraged to report them to the Office of the Inspector General, U.S. Department of Health and Human Services, at 1-800-HHS-TIPS or TIPS.HHS.GOV.
AUTHORITY FOR ISSUANCE OF THE EUA
The Secretary of the Department of Health and Human Services (HHS) declared a public health emergency that justifies the emergency use of drugs and biological products during the COVID-19 pandemic. In response, FDA has issued an EUA for the unapproved product, Janssen COVID-19 Vaccine, for active immunization to prevent COVID-19 in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
FDA first issued this EUA in February 2021, based on Janssen Biotech, Inc.'s request and submitted data.
Although limited scientific information is available, based on the totality of the scientific evidence available to date, it is reasonable to believe that the Janssen COVID-19 Vaccine may be effective for the prevention of COVID-19 in individuals as specified in the Full EUA Prescribing Information.
This EUA for the Janssen COVID-19 Vaccine will end when the Secretary of HHS determines that the circumstances justifying the EUA no longer exist or when there is a change in the approval status of the product such that an EUA is no longer needed.
For additional information about Emergency Use Authorization visit FDA at: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
THE COUNTERMEASURES INJURY COMPENSATION PROGRAM
The Countermeasures Injury Compensation Program (CICP) is a federal program that has been created to help pay for related costs of medical care and other specific expenses to compensate people injured after use of certain medical countermeasures. Medical countermeasures are specific vaccines, medications, devices, or other items used to prevent, diagnose, or treat the public during a public health emergency or a security threat. For more information about CICP, visit www.hrsa.gov/cicp, email cicp@hrsa.gov, or call: 1-855-266-2427.
Manufactured by:
Janssen Biotech, Inc.
a Janssen Pharmaceutical Company of Johnson & Johnson
Horsham, PA 19044, USA
© 2021 Janssen Pharmaceutical Companies
END SHORT VERSION FACT SHEET
Long Version (Full EUA Prescribing Information) Begins On Next Page
Revised: March/13/2023
| FULL EMERGENCY USE AUTHORIZATION (EUA) PRESCRIBING INFORMATION | |
| JANSSEN COVID-19 VACCINE | |
| FULL EMERGENCY USE AUTHORIZATION (EUA) PRESCRIBING INFORMATION: CONTENTS*
WARNING: THROMBOSIS WITH THROMBOCYTOPENIA SYNDROME (TTS)
|
|
WARNING: THROMBOSIS WITH THROMBOCYTOPENIA SYNDROME
The Janssen COVID-19 Vaccine can cause thrombosis with thrombocytopenia syndrome (TTS) which may be life-threatening [see Warnings and Precautions (5.2)] .
TTS may involve thrombosis at unusual locations for a thrombus (i.e., cerebral vein, visceral artery or vein, extremity artery, central artery or vein) or in an extremity vein or pulmonary artery [see Warnings and Precautions (5.2)] .
Among reported cases of TTS following administration of the Janssen COVID-19 Vaccine, symptoms began approximately one to two weeks after vaccination [see Warnings and Precautions (5.2)] .
Instruct Janssen COVID-19 Vaccine recipients to seek immediate medical attention for shortness of breath, chest pain, leg swelling, persistent abdominal pain, neurological symptoms (including severe or persistent headaches or blurred vision), or petechiae beyond the site of vaccination [see Warnings and Precautions (5.2)] .
The clinical course of TTS following administration of the Janssen COVID-19 Vaccine shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS, the use of heparin may be harmful and alternative treatments may be needed [see Warnings and Precautions (5.2)] .
Do not administer the Janssen COVID-19 Vaccine to individuals with a history of thrombosis with thrombocytopenia following the Janssen COVID-19 Vaccine or any other adenovirus-vectored COVID-19 vaccine [see Contraindications (4.2)] .
1 AUTHORIZED USE
Janssen COVID-19 vaccine is authorized for use under an Emergency Use Authorization (EUA) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
2.1 Preparation for Administration
- The Janssen COVID-19 Vaccine is a colorless to slightly yellow, clear to very opalescent suspension. Visually inspect the Janssen COVID-19 Vaccine vials for particulate matter and discoloration prior to administration. If either of these conditions exists, do not administer the vaccine.
- Before withdrawing each dose of vaccine, carefully mix the contents of the multi-dose vial by swirling gently in an upright position for 10 seconds. Do not shake.
- Each dose is 0.5 mL. Each vial contains five doses. Do not pool excess vaccine from multiple vials.
- The Janssen COVID-19 Vaccine does not contain a preservative. Record the date and time of first use on the Janssen COVID-19 Vaccine vial label. After the first dose has been withdrawn, hold the vial between 2° to 8°C (36° to 46°F) for up to 6 hours or at room temperature (maximally 25°C/77°F) for up to 2 hours. Discard if vaccine is not used within these times.
2.2 Administration
Visually inspect each dose in the dosing syringe prior to administration. The Janssen COVID-19 Vaccine is a colorless to slightly yellow, clear to very opalescent suspension. During the visual inspection,
- verify the final dosing volume of 0.5 mL.
- confirm there are no particulates and that no discoloration is observed.
- do not administer if vaccine is discolored or contains particulate matter.
Administer the Janssen COVID-19 Vaccine intramuscularly.
3 DOSAGE FORMS AND STRENGTHS
Janssen COVID-19 Vaccine is a suspension for intramuscular injection. A single dose is 0.5 mL.
4 CONTRAINDICATIONS
4.1 Severe Allergic Reactions
Do not administer the Janssen COVID-19 Vaccine to individuals with a known history of severe allergic reaction (e.g., anaphylaxis) to any component of the Janssen COVID-19 Vaccine [see Description (13)] .
4.2 Thrombosis with Thrombocytopenia
Do not administer the Janssen COVID-19 Vaccine to individuals with a history of thrombosis with thrombocytopenia following the Janssen COVID-19 Vaccine or any other adenovirus-vectored COVID-19 vaccine (e.g., AstraZeneca's COVID-19 vaccine which is not authorized or approved in the United States) [see Warnings and Precautions (5.2)] .
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of the Janssen COVID-19 Vaccine.
Monitor Janssen COVID-19 Vaccine recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention guidelines ( https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
5.2 Thrombosis with Thrombocytopenia Syndrome (TTS)
Reports to the Vaccine Adverse Events Reporting System (VAERS), a passive surveillance system, provide evidence for an increased risk of thrombosis with thrombocytopenia syndrome (TTS) with onset of symptoms approximately one to two weeks after administration of the Janssen COVID-19 Vaccine.
An analysis of VAERS reports of TTS following the receipt of the Janssen COVID-19 Vaccine used the following case definition:
- a thrombosis in an unusual location for a thrombus (i.e., cerebral vein, visceral artery or vein, extremity artery, central artery or vein) and new-onset thrombocytopenia (i.e., platelet count <150,000/μL) occurring any time after vaccination;
or - new-onset thrombocytopenia (i.e., platelet count <150,000/μL), thrombosis in an extremity vein or pulmonary artery in the absence of thrombosis at an unusual location, and a positive anti-PF4 antibody ELISA test or functional HIT (heparin-induced thrombocytopenia) platelet test occurring any time after vaccination.
Cases of TTS following administration of the Janssen COVID-19 Vaccine have been reported in males and females, in a wide age range of individuals 18 years and older, with the highest reporting rate (approximately 8 cases per 1,000,000 doses administered) in females ages 30–49 years; overall, approximately 15% of TTS cases have been fatal. The clinical course of these events shares features with autoimmune heparin-induced thrombocytopenia. Specific risk factors for TTS following administration of the Janssen COVID-19 Vaccine and the level of potential excess risk due to vaccination are under investigation. Currently available evidence supports a causal relationship between TTS and the Janssen COVID-19 Vaccine.
Healthcare professionals should be alert to the signs and symptoms of TTS in individuals who receive the Janssen COVID-19 Vaccine. In individuals with suspected TTS following administration of the Janssen COVID-19 Vaccine, the use of heparin may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.
The American Society of Hematology has published considerations relevant to the diagnosis and treatment of TTS following administration of the Janssen COVID-19 Vaccine ( https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia).
Recipients of Janssen COVID-19 Vaccine should be instructed to seek immediate medical attention if they develop shortness of breath, chest pain, leg swelling, persistent abdominal pain, neurological symptoms (including severe or persistent headaches or blurred vision), or petechiae beyond the site of vaccination.
5.3 Immune Thrombocytopenia (ITP)
Reports of adverse events following use of the Janssen COVID-19 Vaccine under emergency use authorization suggest an increased risk of immune thrombocytopenia (ITP) during the 42 days following vaccination. Individuals with a history of ITP should discuss with their healthcare provider the risk of ITP and the potential need for platelet monitoring following vaccination with the Janssen COVID-19 Vaccine.
5.4 Guillain-Barré Syndrome
Reports of adverse events following use of the Janssen COVID-19 Vaccine under emergency use authorization suggest an increased risk of Guillain-Barré syndrome during the 42 days following vaccination.
5.5 Myocarditis and Pericarditis
Reports of adverse events following use of the Janssen COVID-19 Vaccine under emergency use authorization suggest increased risks of myocarditis and pericarditis, particularly within the period 0 through 7 days following vaccination.
The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis ( https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#myocarditis-pericarditis).
5.6 Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines. Procedures should be in place to avoid injury from fainting.
6 OVERALL SAFETY SUMMARY
It is MANDATORY for vaccination providers to report to the Vaccine Adverse Event Reporting System (VAERS) all vaccine administration errors, all serious adverse events, cases of myocarditis, cases of pericarditis, cases of Multisystem Inflammatory Syndrome (MIS) in adults, and hospitalized or fatal cases of COVID-19 following vaccination with the Janssen COVID-19 Vaccine. To the extent feasible, provide a copy of the VAERS form to Janssen Biotech, Inc. Please see the REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS section for details on reporting to VAERS or Janssen Biotech, Inc.
Adverse Reactions in Clinical Trials
In study COV3001, the most common local solicited adverse reaction (≥10%) reported was injection site pain (48.6%). The most common systemic adverse reactions (≥10%) were headache (38.9%), fatigue (38.2%), myalgia (33.2%), and nausea (14.2%) (see Tables 1 to 4).
Severe allergic reactions, including anaphylaxis, have been reported following administration of the Janssen COVID-19 vaccine.
Adverse Reactions Identified during Post Authorization Use
Anaphylaxis and other severe allergic reactions, thrombosis with thrombocytopenia, immune thrombocytopenia, Guillain-Barré syndrome, myocarditis, pericarditis, and capillary leak syndrome have been reported following administration of the Janssen COVID-19 Vaccine during mass vaccination outside of clinical trials.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Safety Primary vaccination
The safety of the Janssen COVID-19 Vaccine has been assessed in an ongoing Phase 3 Study, COV3001 (NCT04505722) (Study 1). A total of 43,783 individuals were enrolled in this study, of whom 21,895 adults aged 18 years and older received the Janssen COVID-19 Vaccine [Full Analysis Set (FAS)]. This study is being conducted in the United States (n=19,302), Brazil (n=7,278), South Africa (n=6,576), Colombia (n=4,248), Argentina (n=2,996), Peru (n=1,771), Chile (n=1,133), Mexico (n=479). In this study, 45.0% were female, 54.9% were male, 58.7% were White, 19.4% were Black or African American, 45.3% were Hispanic or Latino, 3.3% were Asian, 9.5% were American Indian/Alaska Native and 0.2% were Native Hawaiian or other Pacific Islander, 5.6% were from multiple racial groups and 1.4% were unknown races (see Table 5). The median age of individuals was 52.0 years (range: 18–100). There were 4,217 (9.6%) individuals who were SARS-CoV-2 seropositive at baseline and who were included in the study. In the United States, 838 of 19,302 (4.3%) individuals were SARS-CoV-2 seropositive. Demographic characteristics were similar among individuals who received the Janssen COVID-19 Vaccine and those who received saline placebo.
The safety subset includes 6,736 individuals (3,356 from the Janssen COVID-19 Vaccine group, 3,380 from the placebo group). The demographic profile in the safety subset was similar in terms of age and gender compared to the FAS. A larger percentage of individuals in the safety subset were White (83.4%) compared to the FAS (58.7%). Geographically, the safety subset was limited to individuals from the United States (51.4%), Brazil (38.5%) and South Africa (10.2%). Fewer individuals in the safety subset compared to the FAS were SARS-CoV-2 seropositive at baseline, 4.5% vs. 9.6%, and had at least one comorbidity 34.1% vs 40.8%.
Safety monitoring in the clinical study consisted of monitoring for: (1) solicited local and systemic reactions occurring in the 7 days following vaccination in a subset of individuals (safety subset), (2) unsolicited adverse events (AEs) occurring in the 28 days following vaccination in the safety subset, (3) medically-attended AEs (MAAEs) occurring in the 6 months following vaccination in the entire study population (FAS), (4) serious AEs (SAEs) and AEs leading to study discontinuation for the duration of the study in the entire study population.
Solicited adverse reactions
Shown below are the frequencies of solicited local adverse reactions (Tables 1 and 2) and systemic adverse reactions (Tables 3 and 4) reported in adults by age group in the 7 days following vaccination in Study 1.
| Adverse Reactions | Janssen COVID-19 Vaccine
N=2,036 n(%) | Placebo
N=2,049 n(%) |
|---|---|---|
|
|
||
| Injection Site Pain | ||
| Any | 1,193 (58.6) | 357 (17.4) |
| Grade 3 * | 8 (0.4) | 0 |
| Injection Site Erythema | ||
| Any (≥25 mm) | 184 (9.0) | 89 (4.3) |
| Grade 3 † | 6 (0.3) | 2 (0.1) |
| Injection Site Swelling | ||
| Any (≥25 mm) | 142 (7.0) | 32 (1.6) |
| Grade 3 † | 5 (0.2) | 2 (0.1) |
| Adverse Reactions | Janssen COVID-19 Vaccine
N=1,320 n(%) | Placebo
N=1,331 n(%) |
|---|---|---|
|
|
||
| Injection Site Pain | ||
| Any | 439 (33.3) | 207 (15.6) |
| Grade 3 * | 3 (0.2) | 2 (0.2) |
| Injection Site Erythema | ||
| Any (≥25 mm) | 61 (4.6) | 42 (3.2) |
| Grade 3 † | 1 (0.1) | 0 |
| Injection Site Swelling | ||
| Any (≥25 mm) | 36 (2.7) | 21 (1.6) |
| Grade 3 † | 2 (0.2) | 0 |
| Adverse Reactions | Janssen COVID-19 Vaccine
N=2,036 n(%) | Placebo
N=2,049 n(%) |
|---|---|---|
|
|
||
| Headache | ||
| Any | 905 (44.4) | 508 (24.8) |
| Grade 3 * | 18 (0.9) | 5 (0.2) |
| Fatigue | ||
| Any | 891 (43.8) | 451 (22.0) |
| Grade 3 † | 25 (1.2) | 4 (0.2) |
| Myalgia | ||
| Any | 796 (39.1) | 248 (12.1) |
| Grade 3 † | 29 (1.4) | 1 (<0.1) |
| Nausea | ||
| Any | 315 (15.5) | 183 (8.9) |
| Grade 3 † | 3 (0.1) | 3 (0.1) |
| Fever‡ | ||
| Any | 261 (12.8) | 14 (0.7) |
| Grade 3 | 7 (0.3) | 0 |
| Use of antipyretic or pain medication | 538 (26.4) | 123 (6.0) |
| Adverse Reactions | Janssen COVID-19 Vaccine
N=1,320 n(%) | Placebo
N=1,331 n(%) |
|---|---|---|
|
|
||
| Headache | ||
| Any | 401 (30.4) | 294 (22.1) |
| Grade 3 * | 5 (0.4) | 4 (0.3) |
| Fatigue | ||
| Any | 392 (29.7) | 277 (20.8) |
| Grade 3 † | 10 (0.8) | 5 (0.4) |
| Myalgia | ||
| Any | 317 (24.0) | 182 (13.7) |
| Grade 3 † | 3 (0.2) | 5 (0.4) |
| Nausea | ||
| Any | 162 (12.3) | 144 (10.8) |
| Grade 3 † | 3 (0.2) | 3 (0.2) |
| Fever‡ | ||
| Any | 41 (3.1) | 6 (0.5) |
| Grade 3 | 1 (0.1) | 0 |
| Use of antipyretic or pain medication | 130 (9.8) | 68 (5.1) |
Solicited local and systemic adverse reactions reported following administration of the Janssen COVID-19 Vaccine had a median duration of 1 to 2 days.
Unsolicited adverse events
Individuals within the safety subset in Study 1 (N=6,736) were monitored for unsolicited adverse events (AEs) for 28 days following vaccination with 99.9% (N= 6,730) of individuals completing the full 28 days of follow-up. The proportion of individuals who reported one or more unsolicited AEs was similar among those in the Janssen COVID-19 Vaccine group (13.1%) and those in the placebo group (12.0%).
Serious Adverse Events (SAEs) and other events of interest
In Study 1, up to a cut-off date of January 22, 2021, 54.6% of individuals had follow-up duration of 8 weeks. The median follow-up duration for all individuals was 58 days. SAEs, excluding those related to confirmed COVID-19, were reported by 0.4% (n=83) of individuals who received the Janssen COVID-19 Vaccine (N= 21,895) and 0.4% (n=96) of individuals who received placebo (N= 21,888).
Additional adverse events of interest, including but not limited to allergic, neurologic, inflammatory, vascular, and autoimmune disorders, were analyzed among all adverse events collected through protocol-specified safety monitoring procedures as well as unsolicited reporting.
Urticaria (all non-serious) was reported in five vaccinated individuals and 1 individual who received placebo in the 7 days following vaccination. In addition, an SAE of hypersensitivity, not classified as anaphylaxis, was reported in 1 vaccinated individual with urticaria beginning two days following vaccination and angioedema of the lips with no respiratory distress beginning four days following vaccination. The event was likely related to the vaccine.
An SAE of severe pain in the injected arm, not responsive to analgesics, with immediate onset at time of vaccination, and that was ongoing 74 days following vaccination was reported in an individual who received the Janssen COVID-19 Vaccine. An SAE of severe generalized weakness, fever, and headache, with onset on the day following vaccination and resolution three days following vaccination was reported in an individual who received the Janssen COVID-19 Vaccine. Both SAEs are likely related to the vaccine.
Numerical imbalances, with more events in vaccine than placebo recipients, were observed for the following serious and other adverse events of interest in individuals receiving the vaccine or placebo, respectively:
- Thromboembolic events:
- Deep vein thrombosis: 6 events (2 serious; 5 within 28 days of vaccination) vs. 2 events (1 serious; 2 within 28 days of vaccination).
- Pulmonary embolism: 4 events (3 serious; 2 within 28 days of vaccination) vs. 1 event (serious and within 28 days of vaccination).
- Transverse sinus thrombosis with thrombocytopenia: 1 event (serious, with onset of symptoms 8 days post- vaccination) vs. 0.
- Seizures: 4 events (1 serious; 4 within 28 days of vaccination) vs. 1 event (0 serious and 0 within 28 days following vaccination).
- Tinnitus: 6 events (0 serious; 6 within 28 days of vaccination, including 3 within 2 days of vaccination) vs. 0.
For these events, a causal relationship with the Janssen COVID-19 vaccine could not be determined based on Study 1. The assessment of causality was confounded by the presence of underlying medical conditions that may have predisposed individuals to these events. However, post-authorization experience supports a causal relationship with Janssen COVID-19 Vaccine for the event of transverse sinus thrombosis with thrombocytopenia [see Contraindications (4.2), Warnings and Precautions (5.2) and Overall Safety Summary (6.2)] .
There were no additional notable patterns or numerical imbalances between treatment groups for specific categories of serious adverse events (including neurologic, neuro-inflammatory, and cardiovascular events) that would suggest a causal relationship to the Janssen COVID-19 Vaccine.
Booster Dose Following Primary Vaccination with Janssen COVID-19 Vaccine
Overall, in 5 clinical studies conducted in Belgium, Brazil, Colombia, France, Germany, Japan, Netherlands, Philippines, South Africa, Spain, United Kingdom and United States, approximately 9,000 participants have received 2 doses of the Janssen COVID-19 Vaccine, administered at least 2 months apart and approximately 2,700 participants had at least 2 months of safety follow-up after the booster dose.
A randomized, double-blind, placebo-controlled Phase 2 study, COV2001 (NCT04535453) (Study 2), evaluated the frequency and severity of local and systemic adverse reactions within 7 days of administration of a booster dose of the Janssen COVID-19 Vaccine administered approximately 2 months after the primary vaccination in healthy adults 18 through 55 years of age and adults 65 years and older in good or stable health. A total of 141 individuals received at least one dose of the vaccine and 137 received both the primary vaccination and the booster dose at an interval of 2 months. The median age of individuals was 48 years, and 48 individuals (34%) were 65 years of age and older. Data on solicited adverse reactions after the primary vaccination and after a booster dose are shown in Tables 5–8.
Solicited adverse reactions
| Adverse Reactions | Primary Vaccination
N=93 n(%) | Booster Dose
N=89 n(%) |
|---|---|---|
|
|
||
| Injection Site Pain | ||
| Any | 58 (62.4%) | 53 (59.6%) |
| Grade 3 * | 0 | 1 (1.1%) |
| Injection Site Erythema | ||
| Any (≥25 mm) | 1 (1.1%) | 1 (1.1%) |
| Grade 3 † | 0 | 0 |
| Injection Site Swelling | ||
| Any (≥25 mm) | 1 (1.1%) | 0 |
| Grade 3 † | 0 | 0 |
| Adverse Reactions | Primary Vaccination
N=48 n(%) | Booster Dose
N=48 n(%) |
|---|---|---|
|
|
||
| Injection Site Pain | ||
| Any | 17 (35.4%) | 10 (20.8%) |
| Grade 3 * | 0 | 0 |
| Injection Site Erythema | ||
| Any (≥25 mm) | 0 | 0 |
| Grade 3 † | 0 | 0 |
| Injection Site Swelling | ||
| Any (≥25 mm) | 0 | 0 |
| Grade 3 † | 0 | 0 |
| Adverse Reactions | Primary Vaccination
N=93 n(%) | Booster Dose
N=89 n(%) |
|---|---|---|
|
|
||
| Headache | ||
| Any | 49 (52.7%) | 37 (41.6%) |
| Grade 3 * | 2 (2.2%) | 1 (1.1%) |
| Fatigue | ||
| Any | 55 (59.1%) | 46 (51.7%) |
| Grade 3 † | 1 (1.1%) | 0 |
| Myalgia | ||
| Any | 44 (47.3%) | 32 (36.0%) |
| Grade 3 † | 3 (3.2%) | 2 (2.2%) |
| Nausea | ||
| Any | 13 (14.0%) | 9 (10.1%) |
| Grade 3 † | 1 (1.1%) | 0 |
| Fever‡ | ||
| Any | 13 (14.0%) | 5 (5.6%) |
| Grade 3 | 1 (1.1%) | 0 |
| Adverse Reactions | Primary Vaccination
N=48 n(%) | Booster Dose
N=48 n(%) |
|---|---|---|
|
|
||
| Headache | ||
| Any | 9 (18.8%) | 13 (27.1%) |
| Grade 3 * | 0 | 0 |
| Fatigue | ||
| Any | 9 (18.8%) | 16 (33.3%) |
| Grade 3 † | 0 | 0 |
| Myalgia | ||
| Any | 4 (8.3%) | 5 (10.4%) |
| Grade 3 † | 0 | 0 |
| Nausea | ||
| Any | 0 | 1 (2.1%) |
| Grade 3 † | 0 | 0 |
| Fever‡ | ||
| Any | 1 (2.1%) | 0 |
| Grade 3 | 0 | 0 |
Unsolicited adverse events
An overall assessment of Janssen's safety analyses from studies evaluating 2 doses of Janssen COVID-19 Vaccine did not reveal new safety concerns following a booster dose, as compared with adverse reactions reported following the single-dose primary vaccination.
Booster Dose Following Primary Vaccination with Another Authorized or Approved COVID-19 Vaccine
The safety of a Janssen COVID-19 Vaccine booster dose in individuals who completed primary vaccination with another authorized or approved COVID-19 Vaccine (heterologous booster dose) is inferred from the safety of a Janssen COVID-19 Vaccine booster dose administered following completion of Janssen COVID-19 Vaccine primary vaccination (homologous booster dose) and from data from an independent Phase 1/2 open-label clinical trial (NCT04889209) conducted in the United States that evaluated a heterologous booster dose of the Janssen COVID-19 Vaccine. In this study, adults who had completed primary vaccination with a Moderna COVID-19 Vaccine 2-dose series (N=151), a Janssen COVID-19 Vaccine single-dose (N=156), or a Pfizer-BioNTech COVID-19 Vaccine 2-dose series (N=151) at least 12 weeks prior to enrollment and who reported no history of SARS-CoV-2 infection were randomized 1:1:1 to receive a booster dose of one of three vaccines: Moderna COVID-19 Vaccine, Janssen COVID-19 Vaccine, or Pfizer-BioNTech COVID-19 Vaccine. Adverse events were assessed through 28 days after the booster dose. An overall review of adverse reactions reported following the Janssen COVID-19 Vaccine heterologous booster dose did not identify any new safety concerns, as compared with adverse reactions reported following a Janssen COVID-19 Vaccine primary vaccination or homologous booster dose.
6.2 Post Authorization Experience
The following adverse reactions have been identified during post-authorization use of the Janssen COVID-19 Vaccine. Because these reactions are reported voluntarily, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Blood and Lymphatic System Disorders: Thrombosis with thrombocytopenia, Lymphadenopathy, Immune thrombocytopenia.
Cardiac disorders: Myocarditis, Pericarditis.
Ear and labyrinth disorders: Tinnitus.
Gastrointestinal disorders: Diarrhea, Vomiting.
Immune System Disorders: Allergic reactions, including anaphylaxis.
Nervous System Disorders: Guillain-Barré syndrome, Syncope, Paresthesia, Hypoesthesia, Facial Paralysis (including Bell's Palsy).
Vascular Disorders: Capillary leak syndrome, Thrombosis with thrombocytopenia, Venous thromboembolism (with or without thrombocytopenia).
8 REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS
See Overall Safety Summary (Section 6) for additional information.
The vaccination provider enrolled in the federal COVID-19 Vaccination Program is responsible for MANDATORY reporting of the listed events following Janssen COVID-19 Vaccine administration to the Vaccine Adverse Event Reporting System (VAERS):
- Vaccine administration errors whether or not associated with an adverse event,
- Serious adverse events* (irrespective of attribution to vaccination),
- Cases of myocarditis,
- Cases of pericarditis,
- Cases of Multisystem Inflammatory Syndrome (MIS) in adults,
- Cases of COVID-19 that result in hospitalization or death.
* Serious Adverse Events are defined as:
- Death;
- A life-threatening adverse event;
- Inpatient hospitalization or prolongation of existing hospitalization;
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions;
- A congenital anomaly/birth defect;
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent one of the outcomes listed above.
Instructions for Reporting to VAERS
The vaccination provider enrolled in the federal COVID-19 Vaccination Program should complete and submit a VAERS form to FDA using one of the following methods:
- Complete and submit the report online: https://vaers.hhs.gov/reportevent.html, or
- If you are unable to submit this form electronically, you may fax it to VAERS at 1-877-721-0366. If you need additional help submitting a report you may call the VAERS toll-free information line at 1-800-822-7967 or send an email to info@vaers.org.
IMPORTANT: When reporting adverse events or vaccine administration errors to VAERS, please complete the entire form with detailed information. It is important that the information reported to FDA be as detailed and complete as possible. Information to include:
- Patient demographics, (e.g., patient name, date of birth),
- Pertinent medical history,
- Pertinent details regarding admission and course of illness,
- Concomitant medications,
- Timing of adverse event(s) in relationship to administration of Janssen COVID-19 vaccine,
- Pertinent laboratory and virology information,
- Outcome of the event and any additional follow-up information if it is available at the time of the VAERS report. Subsequent reporting of follow-up information should be completed if additional details become available.
The following steps are highlighted to provide the necessary information for safety tracking:
- In Box 17, provide information on Janssen COVID-19 Vaccine and any other vaccines administered on the same day; and in Box 22, provide information on any other vaccines received within one month prior.
- In Box 18, description of the event:
- Write "Janssen COVID-19 Vaccine EUA" as the first line.
- Provide a detailed report of vaccine administration error and/or adverse event. It is important to provide detailed information regarding the patient and adverse event/medication error for ongoing safety evaluation of this unapproved vaccine. Please see information to include listed above.
- Contact information:
- In Box 13, provide the name and contact information of the prescribing healthcare provider or institutional designee who is responsible for the report.
- In Box 14, provide the name and contact information of the best doctor/healthcare professional to contact about the adverse event.
- In Box 15, provide the address of the facility where vaccine was given (NOT the healthcare provider's office address).
Other Reporting Instructions
Vaccination providers may report to VAERS other adverse events that are not required to be reported using the contact information above.
To the extent feasible, report adverse events to Janssen Biotech, Inc. using the contact information below or by providing a copy of the VAERS form to Janssen Biotech, Inc:
| Fax number | Telephone numbers | |
|---|---|---|
| JNJvaccineAE@its.jnj.com | 215-293-9955 | US Toll Free: 1-800-565-4008
US Toll: (908) 455-9922 |
10 DRUG INTERACTIONS
There are no data to assess the concomitant administration of the Janssen COVID-19 Vaccine with other vaccines.
11 USE IN SPECIFIC POPULATIONS
11.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Janssen COVID-19 Vaccine during pregnancy. Women who are vaccinated with Janssen COVID-19 Vaccine during pregnancy are encouraged to enroll in the registry by visiting https://c-viper.pregistry.com.
Risk Summary
All Pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Available data on Janssen COVID-19 Vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
In a reproductive developmental toxicity study female rabbits were administered 1 mL of the Janssen COVID-19 Vaccine (a single human dose is 0.5 mL) by intramuscular injection 7 days prior to mating and on Gestation Days 6 and 20 (i.e., one vaccination during early and late gestation, respectively). No vaccine related adverse effects on female fertility, embryo-fetal or postnatal development up to Postnatal Day 28 were observed.
11.3 Pediatric Use
Emergency Use Authorization of the Janssen COVID-19 Vaccine does not include use in individuals younger than 18 years of age.
11.4 Geriatric Use
Clinical studies of Janssen COVID-19 Vaccine included individuals 65 years of age and older and their data contributes to the overall assessment of safety and efficacy [see Overall Safety Summary (6.1) and Clinical Trial Results and Supporting Data for EUA (18)] . Of the 21,895 individuals who received a single-dose of the Janssen COVID-19 Vaccine in COV3001, 19.5% (n=4,259) were 65 years of age and older and 3.7% (n=809) were 75 years of age and older. No overall differences in safety or efficacy were observed between individuals 65 years of age and older and younger individuals.
13 DESCRIPTION
The Janssen COVID-19 Vaccine is a colorless to slightly yellow, clear to very opalescent sterile suspension for intramuscular injection. It contains no visible particulates. The vaccine consists of a replication-incompetent recombinant adenovirus type 26 (Ad26) vector expressing the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike (S) protein in a stabilized conformation.
The Ad26 vector expressing the SARS-CoV-2 S protein is grown in PER.C6 TetR cells, in media containing amino acids and no animal-derived proteins. After propagation, the vaccine is processed through several purification steps, formulated with inactive ingredients and filled into vials.
Each 0.5 mL dose of Janssen COVID-19 Vaccine is formulated to contain 5×10 10 virus particles (VP) and the following inactive ingredients: citric acid monohydrate (0.14 mg), trisodium citrate dihydrate (2.02 mg), ethanol (2.04 mg), 2-hydroxypropyl-β-cyclodextrin (HBCD) (25.50 mg), polysorbate-80 (0.16 mg), sodium chloride (2.19 mg). Each dose may also contain residual amounts of host cell proteins (≤0.15 mcg) and/or host cell DNA (≤3 ng).
Janssen COVID-19 Vaccine does not contain a preservative.
The vial stoppers are not made with natural rubber latex.
14 CLINICAL PHARMACOLOGY
14.1 Mechanism of Action
The Janssen COVID-19 Vaccine is composed of a recombinant, replication-incompetent human adenovirus type 26 vector that, after entering human cells, expresses the SARS-CoV-2 spike (S) antigen without virus propagation. An immune response elicited to the S antigen protects against COVID-19.
18 CLINICAL TRIAL RESULTS AND SUPPORTING DATA FOR EUA
18.1 Efficacy of Primary Vaccination
A primary analysis (cut-off date January 22, 2021) of a multicenter, randomized, double-blind, placebo-controlled Phase 3 Study (Study 1) was conducted in the United States, South Africa, Brazil, Chile, Argentina, Colombia, Peru and Mexico to assess the efficacy, safety, and immunogenicity of a single-dose of the Janssen COVID-19 Vaccine for the prevention of COVID-19 in adults aged 18 years and older. Randomization was stratified by age (18–59 years, 60 years and older) and presence or absence of comorbidities associated with an increased risk of progression to severe COVID-19. The study allowed for the inclusion of individuals with stable pre-existing medical conditions, defined as disease not requiring significant change in therapy during the 3 months preceding vaccination, as well as individuals with stable human immunodeficiency virus (HIV) infection.
A total of 44,325 individuals were randomized equally to receive Janssen COVID-19 Vaccine or saline placebo. Individuals are planned to be followed for up to 24 months, for assessments of safety and efficacy against COVID-19.
The primary efficacy analysis population of 39,321 individuals (19,630 in the Janssen COVID-19 Vaccine group and 19,691 in the placebo group) included 38,059 SARS-CoV-2 seronegative individuals at baseline and 1,262 individuals with an unknown serostatus. Demographic and baseline characteristics were similar among individuals who received the Janssen COVID-19 Vaccine and those who received placebo (see Table 9).
| Janssen COVID-19 Vaccine
(N=19,630) n (%) | Placebo
(N=19,691) n (%) |
|
|---|---|---|
|
|
||
| Sex | ||
| Male | 10,924 (55.6) | 10,910 (55.4) |
| Female | 8,702 (44.3) | 8,777 (44.6) |
| Age (years) | ||
| Mean (SD) | 51.1 (15.0) | 51.2 (15.0) |
| Median | 52.0 | 53.0 |
| Min, max | (18; 100) | (18; 94) |
| Age group | ||
| ≥18 to 59 years of age | 12,830 (65.4) | 12,881 (65.4) |
| ≥60 years of age | 6,800 (34.6) | 6,810 (34.6) |
| ≥65 years of age | 3,984 (20.3) | 4,018 (20.4) |
| ≥75 years of age | 755 (3.8) | 693 (3.5) |
| Race* | ||
| White | 12,200 (62.1) | 12,216 (62.0) |
| Black or African American | 3,374 (17.2) | 3,390 (17.2) |
| Asian | 720 (3.7) | 663 (3.4) |
| American Indian/Alaska Native † | 1,643 (8.4) | 1,628 (8.3) |
| Native Hawaiian or other Pacific Islander | 54 (0.3) | 45 (0.2) |
| Multiple | 1,036 (5.3) | 1,087 (5.5) |
| Unknown | 262 (1.3) | 272 (1.4) |
| Not reported | 341 (1.7) | 390 (2.0) |
| Ethnicity | ||
| Hispanic or Latino | 8,793 (44.8) | 8,936 (45.4) |
| Not Hispanic or Latino | 10,344 (52.7) | 10,259 (52.1) |
| Unknown | 173 (0.9) | 162 (0.8) |
| Not reported | 319 (1.6) | 333 (1.7) |
| Region | ||
| Northern America (United States) | 9,185 (46.8) | 9,171 (46.6) |
| Latin America | 7,967 (40.6) | 8,014 (40.7) |
| Southern Africa (South Africa) | 2,478 (12.6) | 2,506 (12.7) |
| Comorbidities‡ | ||
| Yes | 7,830 (39.9) | 7,867 (40.0) |
| No | 11,800 (60.1) | 11,824 (60.0) |
Efficacy Against COVID-19
The co-primary endpoints evaluated the first occurrence of moderate to severe/critical COVID-19 with onset of symptoms at least 14 days and at least 28 days after vaccination. Moderate to severe/critical COVID-19 was molecularly confirmed by a central laboratory based on a positive SARS-CoV-2 viral RNA result using a polymerase chain reaction (PCR)-based test.
- Moderate COVID-19 was defined based on the following criteria: the individual must have experienced any one of the following new or worsening signs or symptoms: respiratory rate ≥20 breaths/minute, abnormal saturation of oxygen (SpO2) but still >93% on room air at sea level, clinical or radiologic evidence of pneumonia, radiologic evidence of deep vein thrombosis (DVT), shortness of breath or difficulty breathing OR any two of the following new or worsening signs or symptoms: fever (≥38.0°C or ≥100.4°F), heart rate ≥90 beats/minute, shaking chills or rigors, sore throat, cough, malaise, headache, muscle pain (myalgia), gastrointestinal symptoms, new or changing olfactory or taste disorders, red or bruised appearing feet or toes.
- Severe/critical COVID-19 was defined based on the following criteria: the individual must have experienced any one of the following at any time during the course of observation: clinical signs at rest indicative of severe systemic illness (respiratory rate ≥30 breaths/minute, heart rate ≥125 beats/minute, oxygen saturation (SpO2) ≤93% on room air at sea level, or partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) <300 mmHg), respiratory failure (defined as needing high-flow oxygen, non-invasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation [ECMO]), evidence of shock (defined as systolic blood pressure <90 mmHg, diastolic blood pressure <60 mmHg, or requiring vasopressors), significant acute renal, hepatic, or neurologic dysfunction, admission to intensive care unit (ICU), death.
Final determination of severe/critical COVID-19 cases were made by an independent adjudication committee.
Primary analysis
The median length of follow up for efficacy for individuals in the study was 8 weeks post-vaccination. Vaccine Efficacy (VE) for the co-primary endpoints against moderate to severe/critical COVID-19 in individuals who were seronegative or who had an unknown serostatus at baseline was 66.9% (95% CI: 59.0; 73.4) at least 14 days after vaccination and 66.1% (95% CI: 55.0; 74.8) at least 28 days after vaccination (see Table 10).
| Subgroup | Janssen COVID-19 Vaccine
N=19,630 | Placebo
N=19,691 | % Vaccine Efficacy
(95% CI) |
||
|---|---|---|---|---|---|
| COVID-19 Cases
(n) | Person-Years | COVID-19 Cases
(n) | Person-Years | ||
|
|
|||||
| 14 days post-vaccination | |||||
| All subjects* | 116 | 3116.6 | 348 | 3096.1 | 66.9
(59.0; 73.4) |
| 18 to 59 years of age | 95 | 2106.8 | 260 | 2095.0 | 63.7
(53.9; 71.6) |
| 60 years and older | 21 | 1009.8 | 88 | 1001.2 | 76.3
(61.6; 86.0) |
| 28 days post-vaccination | |||||
| All subjects* | 66 | 3102.0 | 193 | 3070.7 | 66.1
(55.0; 74.8) † |
| 18 to 59 years of age | 52 | 2097.6 | 152 | 2077.0 | 66.1
(53.3; 75.8) |
| 60 years and older | 14 | 1004.4 | 41 | 993.6 | 66.2
(36.7; 83.0) |
Vaccine efficacy against severe/critical COVID-19 at least 14 days after vaccination was 76.7% (95% CI: 54.6; 89.1) and 85.4% (95% CI: 54.2; 96.9) at least 28 days after vaccination (see Table 11).
| Subgroup | Janssen COVID-19 Vaccine
N=19,630 | Placebo
N=19,691 | % Vaccine Efficacy
(95% CI) |
||
|---|---|---|---|---|---|
| COVID-19 Cases
(n) | Person-Years | COVID-19 Cases
(n) | Person-Years | ||
|
|
|||||
| 14 days post-vaccination | |||||
| Severe/critical | 76.7 | ||||
| 14 | 3125.1 | 60 | 3122.0 | (54.6; 89.1) * | |
| 28 days post-vaccination | |||||
| Severe/critical | 85.4 | ||||
| 5 | 3106.2 | 34 | 3082.6 | (54.2; 96.9) * | |
Among all COVID-19 cases with onset at least 14 days post vaccination, including cases diagnosed by a positive PCR from a local laboratory and still awaiting confirmation at the central laboratory (as of January 22, 2021), there were 2 COVID-19 related hospitalizations in the vaccine group (with none after 28 days) and 29 in the placebo group (with 16 after 28 days).
As of the primary analysis cut-off date of January 22, 2021, there were no COVID-19-related deaths reported in Janssen COVID-19 Vaccine recipients compared to 5 COVID-19-related deaths reported in placebo recipients, who were SARS-CoV-2 PCR negative at baseline.
Janssen COVID-19 Vaccine Efficacy in Countries With Different Circulating SARS-CoV-2 Variants.
Exploratory subgroup analyses of vaccine efficacy against moderate to severe/critical COVID-19 and severe/critical COVID-19 for Brazil, South Africa, and the United States were conducted (see Table 12). For the subgroup analyses, all COVID-19 cases accrued up to the primary efficacy analysis data cut-off date, including cases confirmed by the central laboratory and cases with documented positive SARS-CoV-2 PCR from a local laboratory which are still awaiting confirmation by the central laboratory, were included. The concordance rate observed up to the data cut-off date between the PCR results from the local laboratory and the central laboratory was 90.3%.
| Onset | Severity | ||
|---|---|---|---|
| Moderate to Severe/Critical
Point estimate (95% CI) | Severe/Critical
Point estimate (95% CI) |
||
| US | at least 14 days after vaccination | 74.4% (65.0; 81.6) | 78.0% (33.1; 94.6) |
| at least 28 days after vaccination | 72.0% (58.2;81.7) | 85.9% (-9.4; 99.7) | |
| Brazil | at least 14 days after vaccination | 66.2% (51.0; 77.1) | 81.9% (17.0; 98.1) |
| at least 28 days after vaccination | 68.1% (48.8; 80.7) | 87.6% (7.8; 99.7) | |
| South Africa | at least 14 days after vaccination | 52.0% (30.3; 67.4) | 73.1% (40.0; 89.4) |
| at least 28 days after vaccination | 64.0% (41.2; 78.7) | 81.7% (46.2; 95.4) | |
Strain sequencing was conducted on available samples with sufficient viral load from centrally confirmed COVID-19 cases (one sequence per case). As of February 12, 2021, samples from 71.7% of central laboratory confirmed primary analysis cases had been sequenced [United States (73.5%), South Africa (66.9%) and Brazil (69.3%)]. In the United States, 96.4% of strains were identified as the Wuhan-H1 variant D614G; in South Africa, 94.5% of strains were identified as the 20H/501Y.V2 variant (B.1.351 lineage); in Brazil, 69.4% of strains were identified to be a variant of the P.2 lineage and 30.6% of strains were identified as the Wuhan-H1 variant D614G. As of February 12, 2021, SARS-CoV-2 variants from the B1.1.7 or P.1 lineages were not found in any of the sequenced samples.
18.2 Immunogenicity of a Booster Dose following Primary Vaccination with Janssen COVID-19 Vaccine
In Study 2, individuals 18 through 55 years of age and 65 years and older received a booster dose of the Janssen COVID-19 Vaccine approximately 2 months after the primary vaccination. Immunogenicity was assessed by measuring neutralizing antibodies to SARS-CoV-2 Victoria/1/2020 strain using a qualified wild-type virus neutralization assay (wtVNA). Immunogenicity data are available from 39 individuals, of whom 15 were 65 years of age and older, and are summarized in Table 13. Based on a limited number of individuals from this study, a similar fold-rise in neutralizing antibody titers from pre-booster to 14 and 28 days post-booster was observed between individuals 18 through 55 years of age and individuals 65 years of age and older.
| Baseline
(Day 1) | 28 Days Post-Primary Vaccination
(Day 29) | Pre-Booster Dose
(Day 57) | 14 Days Post-Booster Dose
(Day 71) | 28 Days Post-Booster Dose
(Day 85) |
|
|---|---|---|---|---|---|
| LLOQ = lower limit of quantification | |||||
|
|
|||||
| N | 38 | 39 | 39 | 39 | 38 |
| Geometric mean titer (95% CI) | <LLOQ (<LLOQ, <LLOQ) | 260 (196, 346) | 212 (142, 314) | 514 (357, 740) | 424 (301, 597) |
| Geometric mean fold increase (95% CI) from baseline | n/a | 4.4 (3.3, 5.7) | 3.7 (2.6, 5.2) | 8.8 (6.2, 12.7) | 7.4 (5.4, 10.2) |
| Geometric mean fold increase (95% CI) from day 29 | n/a | n/a | 0.9 (0.7, 1.1) | 2.0 (1.5, 2.7) | 1.6 (1.2, 2.1) |
| Geometric mean fold increase (95% CI) from pre-booster | n/a | n/a | n/a | 2.3 (1.7, 3.0) | 1.8 (1.4, 2.4) |
When evaluated descriptively among a limited number of study participants using an ELISA assay, increases in anti-Spike protein binding IgG antibodies post booster were not lower for an interval of 6 months compared with an interval of 2 months between primary vaccination and booster dose. These data support the effectiveness of a booster dose when administered at an interval of longer than 2 months after primary vaccination.
18.3 Immunogenicity of a Booster Dose Following Primary Vaccination with Another Authorized or Approved COVID-19 Vaccine
Effectiveness of a Janssen COVID-19 Vaccine booster dose in individuals who completed primary vaccination with another authorized or approved COVID-19 Vaccine (heterologous booster dose) is inferred from immunogenicity data supporting effectiveness of a Janssen COVID-19 Vaccine booster dose administered following completion of Janssen COVID-19 Vaccine primary vaccination and from immunogenicity data from an independent Phase 1/2 open-label clinical trial (NCT04889209) conducted in the United States that evaluated a heterologous booster dose of the Janssen COVID-19 Vaccine. In this study, adults who had completed primary vaccination with a Moderna COVID-19 Vaccine 2-dose series (N=151), a Janssen COVID-19 Vaccine single-dose (N=156), or a Pfizer-BioNTech COVID-19 Vaccine 2-dose series (N=151) at least 12 weeks prior to enrollment and who reported no history of SARS-CoV-2 infection were randomized 1:1:1 to receive a booster dose of one of three vaccines: Moderna COVID-19 Vaccine, Janssen COVID-19 Vaccine, or Pfizer-BioNTech COVID-19 Vaccine. Neutralizing antibody titers, as measured by a pseudovirus neutralization assay using a lentivirus expressing the SARS-CoV-2 Spike protein with D614G mutation, were assessed on Day 1 prior to administration of the booster dose and on Day 15 after the booster dose. A booster response to the Janssen COVID-19 Vaccine was demonstrated regardless of primary vaccination.
19 HOW SUPPLIED/STORAGE AND HANDLING
Janssen COVID-19 Vaccine is supplied in a carton of 10 multi-dose vials (NDC: 59676-580-15). A maximum of 5 doses can be withdrawn from the multi-dose vial.
The storage and handling information in this Fact Sheet supersedes the storage and handling information on the carton and vial labels.
Storage Prior to First Puncture of the Vaccine Vial
Store unpunctured multi-dose vials of the Janssen COVID-19 Vaccine at 2°C to 8°C (36°F to 46°F) and protect from light. Do not store frozen.
Unpunctured vials of Janssen COVID-19 Vaccine may be stored between 9°C to 25°C (47°F to 77°F) for up to 12 hours.
The Janssen COVID-19 Vaccine is initially stored frozen by the manufacturer, then shipped at 2°C to 8°C (36°F to 46°F). If vaccine is still frozen upon receipt, thaw at 2°C to 8°C (36°F to 46°F). If needed immediately, thaw at room temperature (maximally 25°C/77°F). At room temperature (maximally 25°C/77°F), a carton of 10 vials will take approximately 4 hours to thaw, and an individual vial will take approximately 1 hour to thaw. Do not refreeze once thawed.
20 PATIENT COUNSELING INFORMATION
Advise the recipient or caregiver to read the Fact Sheet for Recipients and Caregivers.
Instruct Janssen COVID-19 Vaccine recipients to seek immediate medical attention for shortness of breath, chest pain, leg swelling, persistent abdominal pain, neurological symptoms (including severe or persistent headaches or blurred vision), or petechiae beyond the site of vaccination [see Warnings and Precautions (5.2)] .
In individuals with a history of ITP, discuss the risk of ITP and the potential need for platelet monitoring following vaccination with the Janssen COVID-19 Vaccine [see Warnings and Precautions (5.3)] .
The vaccination provider must include vaccination information in the state/local jurisdiction's Immunization Information System (IIS) or other designated system. Advise recipient or caregiver that more information about IISs can be found at: https://www.cdc.gov/vaccines/programs/iis/about.html.
21 CONTACT INFORMATION
For general questions or to access the most recent Janssen COVID-19 Vaccine Fact Sheets, scan the QR code using your device, visit www.janssencovid19vaccine.com or call the telephone numbers provided below.
| QR Code | Fact Sheets Website | Telephone numbers |
|---|---|---|
|
| www.janssencovid19vaccine.com. | US Toll Free: 1-800-565-4008
US Toll: 1-908-455-9922 |
This Full EUA Prescribing Information may have been updated. For the most recent Full EUA Prescribing Information, please see www.janssencovid19vaccine.com.
Manufactured by:
Janssen Biotech, Inc.
a Janssen Pharmaceutical Company of Johnson & Johnson
Horsham, PA 19044, USA
Revised: March/13/2023
© 2021 Janssen Pharmaceutical Companies
FACT SHEET FOR RECIPIENTS AND CAREGIVERS
EMERGENCY USE AUTHORIZATION (EUA) OF
THE JANSSEN COVID-19 VACCINE TO PREVENT CORONAVIRUS DISEASE 2019
(COVID-19)
The Janssen COVID-19 Vaccine is authorized for use in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and for individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine. The Janssen COVID-19 Vaccine can cause blood clots with low levels of platelets (blood cells that help your body stop bleeding), which may be fatal.
You are being offered the Janssen COVID-19 Vaccine to prevent Coronavirus Disease 2019 (COVID-19) caused by SARS-CoV-2 because there is currently a pandemic of COVID-19.
This Fact Sheet contains information to help you understand the risks and benefits of receiving the Janssen COVID-19 Vaccine.
The Janssen COVID-19 Vaccine may prevent you from getting COVID-19.
Read this Fact Sheet for information about the Janssen COVID-19 Vaccine. Talk to the vaccination provider if you have questions.
The Janssen COVID-19 Vaccine has received EUA from FDA to provide the following doses in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine:
- A single dose primary vaccination.
- A single booster dose after completing a primary vaccination with the Janssen COVID-19 Vaccine.
- A single booster dose after completing primary vaccination with a different authorized or approved COVID-19 vaccine.
The Janssen COVID-19 Vaccine may not protect everyone.
This Fact Sheet may have been updated. For the most recent Fact Sheet, please visit www.janssencovid19vaccine.com.
WHAT YOU NEED TO KNOW BEFORE YOU GET THIS VACCINE
WHAT IS COVID-19?
COVID-19 is caused by a coronavirus called SARS-CoV-2. This type of coronavirus has not been seen before. You can get COVID-19 through contact with another person who has the virus. It is predominantly a respiratory illness that can affect other organs. People with COVID-19 have had a wide range of symptoms reported, ranging from mild symptoms to severe illness. Symptoms may appear 2 to 14 days after exposure to the virus. Common symptoms may include: fever or chills; cough; shortness of breath; fatigue; muscle or body aches; headache; new loss of taste or smell; sore throat; congestion or runny nose; nausea or vomiting; diarrhea.
WHAT IS THE JANSSEN COVID-19 VACCINE?
The Janssen COVID-19 Vaccine is an unapproved vaccine that may prevent COVID-19.
Under an EUA, the FDA has authorized the emergency use of the Janssen COVID-19 Vaccine to prevent COVID-19 in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
For more information on EUA, see the " What is an Emergency Use Authorization (EUA)?" section at the end of this Fact Sheet.
WHAT SHOULD YOU MENTION TO YOUR VACCINATION PROVIDER BEFORE YOU GET THE JANSSEN COVID-19 VACCINE?
Tell the vaccination provider about all of your medical conditions, including if you:
- have any allergies,
- have a fever,
- have a bleeding disorder or are on a blood thinner,
- have ever had a low level of platelets (blood cells that help your body stop bleeding),
- are immunocompromised or are on a medicine that affects your immune system,
- are pregnant or plan to become pregnant,
- are breastfeeding,
- have received another COVID-19 vaccine,
- have ever fainted in association with an injection.
WHO SHOULD GET THE JANSSEN COVID-19 VACCINE?
FDA has authorized the emergency use of the Janssen COVID-19 Vaccine in individuals 18 years of age and older for whom other FDA-authorized or approved COVID-19 vaccines are not accessible or clinically appropriate, and in individuals 18 years of age and older who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
WHO SHOULD NOT GET THE JANSSEN COVID-19 VACCINE?
You should not get the Janssen COVID-19 Vaccine if you:
- had a severe allergic reaction after a previous dose of this vaccine.
- had a severe allergic reaction to any ingredient of this vaccine.
- had a blood clot along with a low level of platelets (blood cells that help your body stop bleeding) following Janssen COVID-19 Vaccine or following AstraZeneca's COVID-19 vaccine (not authorized or approved in the United States).
WHAT ARE THE INGREDIENTS IN THE JANSSEN COVID-19 VACCINE?
The Janssen COVID-19 Vaccine includes the following ingredients: recombinant, replication-incompetent adenovirus type 26 expressing the SARS-CoV-2 spike protein, citric acid monohydrate, trisodium citrate dihydrate, ethanol, 2-hydroxypropyl-β-cyclodextrin (HBCD), polysorbate-80, sodium chloride.
HOW IS THE JANSSEN COVID -19 VACCINE GIVEN?
The Janssen COVID-19 Vaccine will be given to you as an injection into the muscle.
Primary Vaccination
The Janssen COVID-19 Vaccine is administered as a single dose.
Booster Dose
- A single booster dose of the Janssen COVID-19 Vaccine may be administered at least two months after primary vaccination with the Janssen COVID-19 Vaccine.
- A single booster dose of the Janssen COVID-19 Vaccine may be administered after completing primary vaccination with a different authorized or approved COVID-19 vaccine. Please check with your health care provider regarding timing of the booster dose.
HAS THE JANSSEN COVID-19 VACCINE BEEN USED BEFORE?
The Janssen COVID-19 Vaccine is an unapproved vaccine. In clinical trials, more than 61,000 individuals 18 years of age and older have received the Janssen COVID-19 Vaccine. Millions of individuals have received the vaccine under EUA since February 27, 2021.
WHAT ARE THE BENEFITS OF THE JANSSEN COVID-19 VACCINE?
The Janssen COVID-19 Vaccine has been shown to prevent COVID-19. The duration of protection against COVID-19 is currently unknown.
WHAT ARE THE RISKS OF THE JANSSEN COVID-19 VACCINE?
Side effects that have been reported with the Janssen COVID-19 Vaccine include:
- Injection site reactions: pain, redness of the skin and swelling.
- General side effects: headache, feeling very tired, muscle aches, nausea, and fever.
- Swollen lymph nodes.
- Blood clots.
- Unusual feeling in the skin (such as tingling or a crawling feeling) (paresthesia), decreased feeling or sensitivity, especially in the skin (hypoesthesia).
- Persistent ringing in the ears (tinnitus).
- Diarrhea, vomiting.
Severe Allergic Reactions
There is a remote chance that the Janssen COVID-19 Vaccine could cause a severe allergic reaction. A severe allergic reaction would usually occur within a few minutes to one hour after getting a dose of the Janssen COVID-19 Vaccine. For this reason, your vaccination provider may ask you to stay at the place where you received your vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include:
- Difficulty breathing,
- Swelling of your face and throat,
- A fast heartbeat,
- A bad rash all over your body,
- Dizziness and weakness.
Blood Clots with Low Levels of Platelets
Blood clots involving blood vessels in the brain, lungs, abdomen, and legs along with low levels of platelets (blood cells that help your body stop bleeding), have occurred in some people who have received the Janssen COVID-19 Vaccine. In people who developed these blood clots and low levels of platelets, symptoms began approximately one to two weeks after vaccination. Blood clots with low levels of platelets following the Janssen COVID-19 Vaccine have been reported in males and females, across a wide age range of individuals 18 years and older; reporting has been highest in females ages 30 through 49 years (about 8 cases for every 1,000,000 vaccine doses administered), and about 1 out of every 7 cases has been fatal. You should seek medical attention right away if you have any of the following symptoms after receiving the Janssen COVID-19 Vaccine:
- Shortness of breath,
- Chest pain,
- Leg swelling,
- Persistent abdominal pain,
- Severe or persistent headaches or blurred vision,
- Easy bruising or tiny blood spots under the skin beyond the site of the injection.
Immune Thrombocytopenia (ITP)
Immune Thrombocytopenia (ITP) is a disorder that can cause easy or excessive bruising and bleeding due to very low levels of platelets. ITP has occurred in some people who have received the Janssen COVID-19 Vaccine. In most of these people, symptoms began within 42 days following receipt of the Janssen COVID-19 Vaccine. The chance of having this occur is very low. If you have ever had a diagnosis of ITP, talk to your vaccination provider before you get the Janssen COVID-19 Vaccine. You should seek medical attention right away if you develop any of the following symptoms after receiving the Janssen COVID-19 Vaccine:
- Easy or excessive bruising or tiny blood spots under the skin beyond the site of the injection,
- Unusual or excessive bleeding.
Guillain Barré Syndrome
Guillain Barré syndrome (a neurological disorder in which the body's immune system damages nerve cells, causing muscle weakness and sometimes paralysis) has occurred in some people who have received the Janssen COVID-19 Vaccine. In most of these people, symptoms began within 42 days following receipt of the Janssen COVID-19 Vaccine. The chance of having this occur is very low. You should seek medical attention right away if you develop any of the following symptoms after receiving the Janssen COVID-19 Vaccine:
- Weakness or tingling sensations, especially in the legs or arms, that's worsening and spreading to other parts of the body.
- Difficulty walking.
- Difficulty with facial movements, including speaking, chewing, or swallowing.
- Double vision or inability to move eyes.
- Difficulty with bladder control or bowel function.
These may not be all the possible side effects of the Janssen COVID-19 Vaccine. Serious and unexpected effects may occur. The Janssen COVID-19 Vaccine is still being studied in clinical trials.
WHAT SHOULD I DO ABOUT SIDE EFFECTS?
If you experience a severe allergic reaction, call 9-1-1, or go to the nearest hospital.
Call the vaccination provider or your healthcare provider if you have any side effects that bother you or do not go away.
Report vaccine side effects to FDA/CDC Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to https://vaers.hhs.gov/reportevent.html. Please include "Janssen COVID-19 Vaccine EUA" in the first line of box #18 of the report form.
In addition, you can report side effects to Janssen Biotech, Inc. at the contact information provided below.
| Fax number | Telephone numbers | |
|---|---|---|
| JNJvaccineAE@its.jnj.com | 215-293-9955 | US Toll Free: 1-800-565-4008
US Toll: (908) 455-9922 |
You may also be given an option to enroll in v-safe. V-safe is a new voluntary smartphone-based tool that uses text messaging and web surveys to check in with people who have been vaccinated to identify potential side effects after COVID-19 vaccination. V-safe asks questions that help CDC monitor the safety of COVID-19 vaccines. V-safe also provides live telephone follow-up by CDC if participants report a significant health impact following COVID-19 vaccination. For more information on how to sign up, visit: www.cdc.gov/vsafe.
WHAT IF I DECIDE NOT TO GET THE JANSSEN COVID-19 VACCINE?
Under the EUA, there is an option to accept or refuse receiving the vaccine. Should you decide not to receive the Janssen COVID-19 Vaccine, it will not change your standard medical care.
ARE OTHER VACCINES AVAILABLE FOR PREVENTING COVID-19 BESIDES JANSSEN COVID-19 VACCINE?
COMIRNATY and SPIKEVAX are FDA-approved COVID-19 vaccines. Other vaccines to prevent COVID-19 may be available under EUA. The Janssen COVID-19 Vaccine is only authorized if other COVID-19 vaccines are not accessible or clinically appropriate, and for individuals who elect to receive the Janssen COVID-19 Vaccine because they would otherwise not receive a COVID-19 vaccine.
CAN I RECEIVE THE JANSSEN COVID-19 VACCINE AT THE SAME TIME AS OTHER VACCINES?
Data have not yet been submitted to FDA on administration of the Janssen COVID-19 Vaccine at the same time as other vaccines. If you are considering receiving the Janssen COVID-19 Vaccine with other vaccines, discuss your options with your healthcare provider.
WHAT IF I AM PREGNANT OR BREASTFEEDING?
If you are pregnant or breastfeeding, discuss your options with your healthcare provider.
WILL THE JANSSEN COVID-19 VACCINE GIVE ME COVID-19?
No. The Janssen COVID-19 Vaccine does not contain SARS-CoV-2 and cannot give you COVID-19.
KEEP YOUR VACCINATION CARD
When you receive the Janssen COVID-19 Vaccine, you will get a vaccination card to document the name of the vaccine and date of when you received the vaccine.
ADDITIONAL INFORMATION
If you have questions or to access the most recent Janssen COVID-19 Vaccine Fact Sheets, scan the QR code using your device, visit the website or call the telephone numbers provided below.
| QR Code | Fact Sheets Website | Telephone numbers |
|---|---|---|
|
| www.janssencovid19vaccine.com. | US Toll Free: 1-800-565-4008
US Toll: (908) 455-9922 |
HOW CAN I LEARN MORE?
- Ask the vaccination provider.
- Visit CDC at https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- Visit FDA at https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
Contact your local or state public health department.
WHERE WILL MY VACCINATION INFORMATION BE RECORDED?
The vaccination provider may include your vaccination information in your state/local jurisdiction's Immunization Information System (IIS) or other designated system. For more information about IISs visit: https://www.cdc.gov/vaccines/programs/iis/about.html.
CAN I BE CHARGED AN ADMINISTRATION FEE FOR RECEIPT OF THE COVID-19 VACCINE?
No. At this time, the provider cannot charge you for a vaccine dose and you cannot be charged an out-of-pocket vaccine administration fee or any other fee if only receiving a COVID-19 vaccination. However, vaccination providers may seek appropriate reimbursement from a program or plan that covers COVID-19 vaccine administration fees for the vaccine recipient (private insurance, Medicare, Medicaid, HRSA COVID-19 Uninsured Program for non-insured recipients).
WHERE CAN I REPORT CASES OF SUSPECTED FRAUD?
Individuals becoming aware of any potential violations of the CDC COVID-19 Vaccination Program requirements are encouraged to report them to the Office of the Inspector General, U.S. Department of Health and Human Services, at 1-800-HHS-TIPS or TIPS.HHS.GOV.
WHAT IS THE COUNTERMEASURE INJURY COMPENSATION PROGRAM?
The Countermeasures Injury Compensation Program (CICP) is a federal program that may help pay for costs of medical care and other specific expenses for certain people who have been seriously injured by certain medicines or vaccines, including this vaccine. Generally, a claim must be submitted to the CICP within one (1) year from the date of receiving the vaccine. To learn more about this program, visit www.hrsa.gov/cicp or call 1-855-266-2427.
WHAT IS AN EMERGENCY USE AUTHORIZATION (EUA)?
The United States FDA has made the Janssen COVID-19 Vaccine available under an emergency access mechanism called an EUA. The EUA is supported by a Secretary of Health and Human Services (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic.
The Janssen COVID-19 Vaccine has not undergone the same type of review as an FDA-approved or cleared product. FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, and available alternatives. In addition, the FDA decision is based on the totality of scientific evidence available showing that the product may be effective to prevent COVID-19 during the COVID-19 pandemic and that the known and potential benefits of the product outweigh the known and potential risks of the product. All of these criteria must be met to allow for the product to be used during the COVID-19 pandemic.
The EUA for the Janssen COVID-19 Vaccine is in effect for the duration of the COVID-19 declaration justifying emergency use of these products, unless terminated or revoked (after which the products may no longer be used).
Manufactured by:
Janssen Biotech, Inc.
a Janssen Pharmaceutical Company of Johnson & Johnson
Horsham, PA 19044, USA
© 2021 Janssen Pharmaceutical Companies
For more information, call US Toll Free: 1-800-565-4008, US Toll: (908) 455-9922 or go to www.janssencovid19vaccine.com
Revised: May/05/2022
PRINCIPAL DISPLAY PANEL - 2.5 mL Vial Label
AW_177786
For use
under
Emergency
Use
Authorization
Janssen COVID-19
Vaccine
5 doses of 0.5 mL
Intramuscular Injection
No Preservative
For EXP: www.vaxcheck.jnj
After first use, hold at
2°C to 8°C (36°F to 46°F).
Discard after 6 hours.
Record date and time
of first use:
LOT

PRINCIPAL DISPLAY PANEL - 10 Vial Carton
NDC: 59676-580-15
No Preservative
10 Multi-dose Vials
Each vial contains 5 doses of 0.5 mL
Janssen COVID-19 Vaccine
SUSPENSION FOR INTRAMUSCULAR INJECTION
For use under Emergency Use Authorization
Attention: After first use, hold at 2°C to 8°C (36°F to 46°F)
for up to 6 hours or at room temperature (maximally
25°C/77°F) for up to 2 hours. Discard if not used within
these times. Do not freeze.
janssen
| JANSSEN COVID-19 VACCINE
ad26.cov2.s injection, suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Janssen Products, LP (804684207) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Grand River Aseptic Manufacturing, Inc. | 005593490 | manufacture(59676-580) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Packaging Coordinators, LLC | 078525133 | pack(59676-580) , label(59676-580) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Indiana, LLC | 172209277 | manufacture(59676-580) , pack(59676-580) , analysis(59676-580) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jannsen Biologics B.V. | 409612918 | api manufacture(59676-580) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen | 415960780 | api manufacture(59676-580) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Emergent Manufacturing Operations Baltimore LLC | 968316831 | manufacture(59676-580) , analysis(59676-580) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.