BYDUREON BCISE- exenatide injection, suspension, extended release
BYDUREON BCise by
Drug Labeling and Warnings
BYDUREON BCise by is a Prescription medication manufactured, distributed, or labeled by AstraZeneca Pharmaceuticals LP, AstraZeneca PLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BYDUREON BCISE safely and effectively. See full prescribing information for BYDUREON BCISE.
BYDUREON BCISE® (exenatide extended-release) injectable suspension, for subcutaneous use.
Initial U.S. Approval: 2005WARNING: RISK OF THYROID C-CELL TUMORS
See full prescribing information for complete boxed warning.
- Exenatide extended-release causes thyroid C-cell tumors at clinically relevant exposures in rats. It is unknown whether BYDUREON BCISE causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC) in humans, as the human relevance of exenatide extended-release-induced rodent thyroid C-cell tumors has not been determined. (5.1, 13.1)
- BYDUREON BCISE is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk of MTC and the symptoms of thyroid tumors. (4, 5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
BYDUREON BCISE is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1, 14)
Limitations of Use:
- Not recommended as first-line therapy for patients inadequately controlled on diet and exercise. (1)
- Should not be used to treat type 1 diabetes or diabetic ketoacidosis. (1)
- Use with prandial insulin has not been studied. (1)
- BYDUREON BCISE is an extended-release formulation of exenatide. Do not coadminister with other exenatide containing products. (1)
- Has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis. (1, 5.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
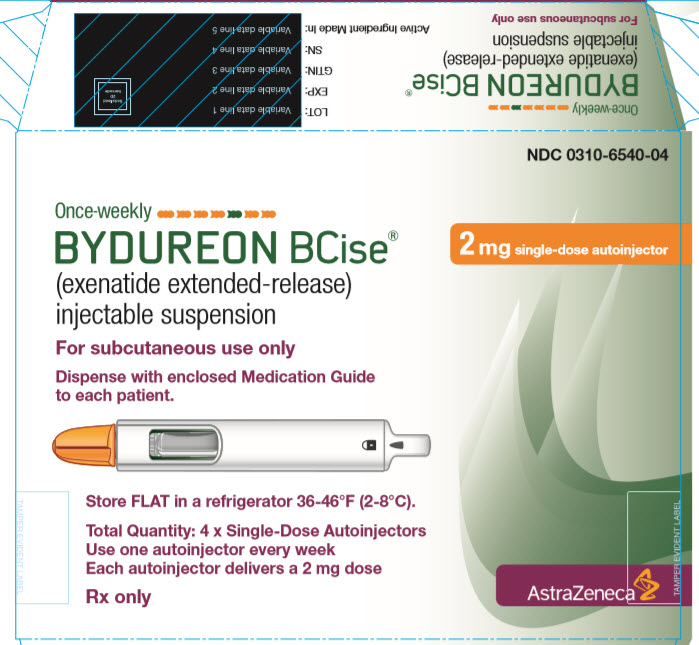
Extended-release injectable suspension: 2 mg of exenatide in a 0.85 mL single-dose autoinjector. (3)
CONTRAINDICATIONS
- Personal or family history of medullary thyroid carcinoma or in patients with Multiple Endocrine Neoplasia syndrome type 2. (4)
- Prior serious hypersensitivity reaction to exenatide or any of the product components. (4)
- History of drug-induced immune-mediated thrombocytopenia from exenatide products (4).
WARNINGS AND PRECAUTIONS
- Acute Pancreatitis: Including fatal and non-fatal hemorrhagic or necrotizing pancreatitis has been reported. Discontinue promptly if pancreatitis is suspected. Do not restart if pancreatitis is confirmed. Consider other antidiabetic therapies if patient has history of pancreatitis. (5.2)
- Hypoglycemia: When used in combination with an insulin secretagogue (e.g., a sulfonylurea) or insulin, consider lowering dose of the secretagogue or insulin to reduce risk of hypoglycemia. (5.3)
- Acute Kidney Injury: May induce nausea and vomiting with transient hypovolemia and may worsen renal function. Postmarketing increased serum creatinine, renal impairment, worsened chronic renal failure and acute renal failure, sometimes requiring hemodialysis or kidney transplantation has been reported. Not recommended for use in patients with eGFR below 45 mL/min/1.73 m2. (5.4, 8.6, 12.3)
- Gastrointestinal Disease: Not recommended in patients with severe gastrointestinal disease (e.g., gastroparesis). (5.5)
- Immunogenicity: Patients may develop antibodies to exenatide. If there is worsening glycemic control or failure to achieve target glycemic control, consider alternative antidiabetic therapy. (5.6)
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis and angioedema) have been reported. Discontinue BYDUREON BCISE and promptly seek medical advice. (5.7)
- Drug-induced Immune-mediated Thrombocytopenia: Serious bleeding which may be fatal has been reported. Discontinue BYDUREON BCISE promptly and avoid re-exposure to exenatide. (5.8)
- Injection-site Reactions: Serious injection-site reactions with or without subcutaneous nodules have been reported. (5.9)
- Acute Gallbladder Disease: If cholelithiasis or cholecystitis are suspected, gallbladder studies are indicated. (5.10)
ADVERSE REACTIONS
Most common (≥5%) in clinical trials: injection-site nodule, nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 and www.bydureonbcise.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pregnancy: Use during pregnancy only if the potential benefit justifies the risk to the fetus. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF THYROID C-CELL TUMORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Missed Dose
2.3 Administration Instructions
2.4 Initiating BYDUREON BCISE Therapy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Thyroid C-cell Tumors
5.2 Acute Pancreatitis
5.3 Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
5.4 Acute Kidney Injury
5.5 Gastrointestinal Disease
5.6 Immunogenicity
5.7 Hypersensitivity
5.8 Drug-Induced Thrombocytopenia
5.9 Injection-Site Reactions
5.10 Acute Gallbladder Disease
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Glycemic Control Trials in Adults with Type 2 Diabetes Mellitus
14.2 EXSCEL Cardiovascular Outcomes Trial in Patients with Type 2 Diabetes
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF THYROID C-CELL TUMORS
- Exenatide extended-release causes an increased incidence in thyroid C-cell tumors at clinically relevant exposures in rats compared to controls. It is unknown whether BYDUREON BCISE causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of exenatide extended-release-induced rodent thyroid C-cell tumors has not been determined [see Warnings and Precautions (5.1) and Nonclinical Toxicology (13.1)].
- BYDUREON BCISE is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of BYDUREON BCISE and inform them of symptoms of thyroid tumors (e.g., mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for detection of MTC in patients treated with BYDUREON BCISE [see Contraindications (4) and Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
BYDUREON BCISE is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [see Clinical Studies (14)].
Limitations of Use
- BYDUREON BCISE is not recommended as first-line therapy for patients who have inadequate glycemic control on diet and exercise because of the uncertain relevance of the rat thyroid C-cell tumor findings to humans [see Warnings and Precautions (5.1)].
- BYDUREON BCISE is not a substitute for insulin. BYDUREON BCISE is not indicated for use in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis, as it would not be effective in these settings.
- The concurrent use of BYDUREON BCISE with prandial insulin has not been studied [see Clinical Studies (14)].
- BYDUREON BCISE is an extended-release formulation of exenatide. BYDUREON BCISE should not be used with other products containing the active ingredient exenatide.
- BYDUREON BCISE has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis [see Warnings and Precautions (5.2) and Adverse Reactions (6.3)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
The recommended dose of BYDUREON BCISE is 2 mg subcutaneously once every 7 days (weekly). The dose can be administered at any time of day, with or without meals.
The day of weekly administration can be changed if necessary, as long as the last dose was administered 3 or more days before the new day of administration.
2.2 Missed Dose
If a dose is missed, administer the dose as soon as noticed, provided the next regularly scheduled dose is due at least 3 days later. Thereafter, patients can resume their usual dosing schedule of once every 7 days (weekly).
If a dose is missed and the next regularly scheduled dose is due 1 or 2 days later, do not administer the missed dose and instead resume BYDUREON BCISE with the next regularly scheduled dose.
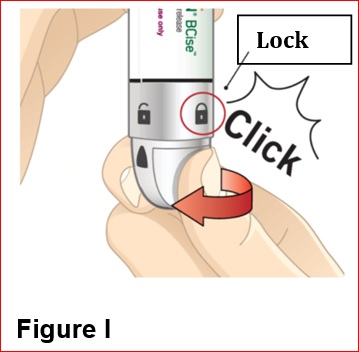
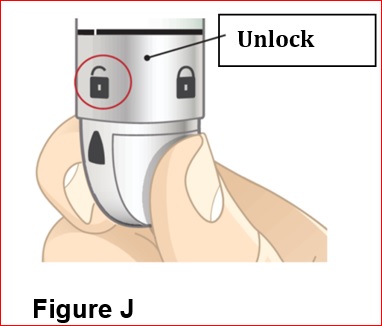
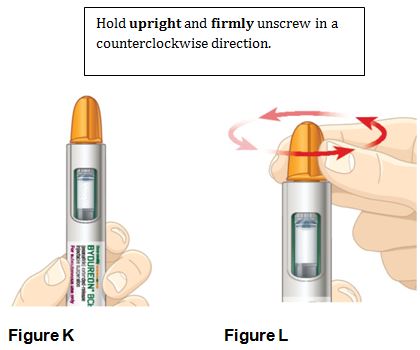
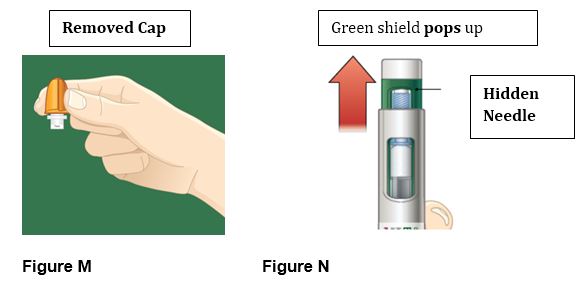
2.3 Administration Instructions
- BYDUREON BCISE is intended for patient self-administration. Prior to initiation, train patients on proper mixing and injection technique to ensure the product is adequately mixed and a full dose is delivered [see Instructions for Use].
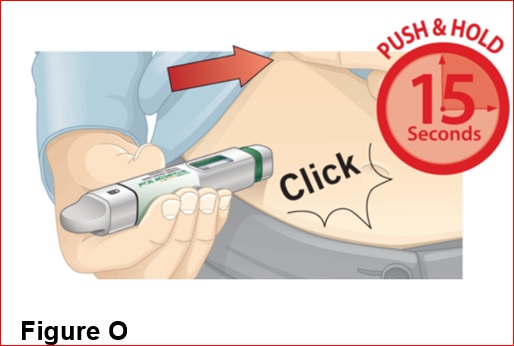
- Remove the autoinjector from the refrigerator 15 minutes prior to mixing the injection, in order to reach room temperature.
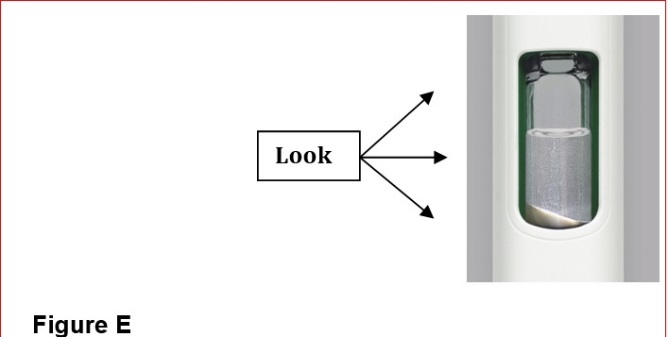
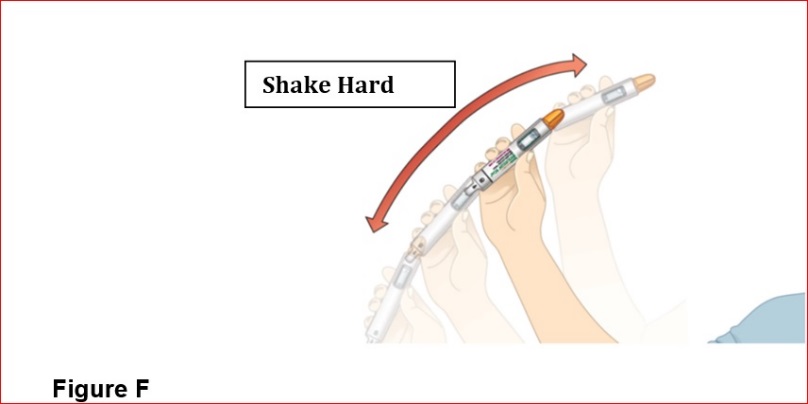
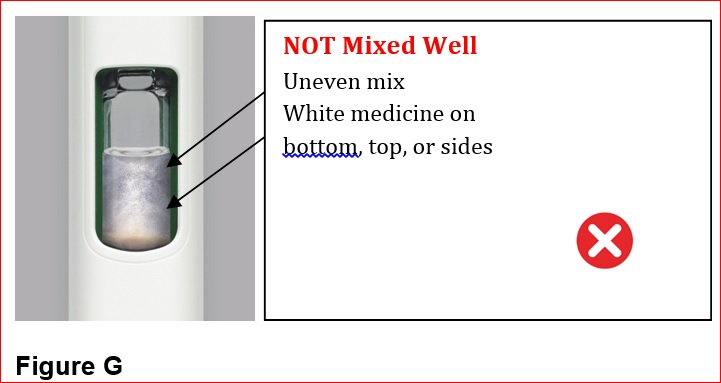
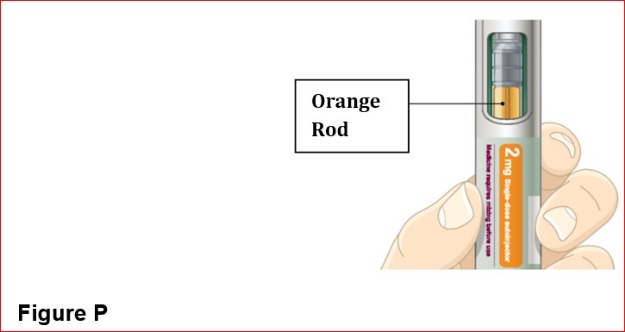
- Mix by shaking vigorously for at least 15 seconds. After mixing, BYDUREON BCISE should appear as an opaque, white to off-white suspension, evenly mixed with no residual medicine along the side, bottom or top of the inspection window.
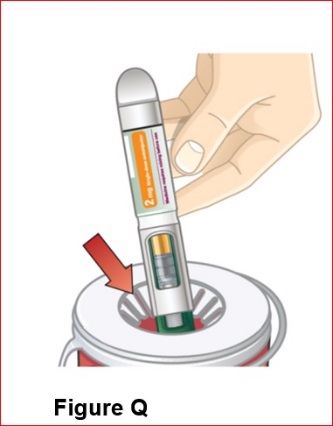
- Inspect visually for particulate matter and discoloration prior to administration (BYDUREON BCISE contains microspheres which appear as white to off-white particles). Do not use if foreign particulate matter is present or if discoloration is observed. Refer patients to the accompanying Instructions for Use for disposal information [see Instructions for Use].
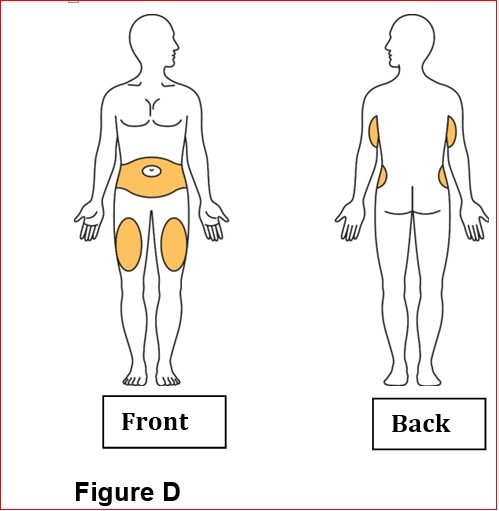
- Administer BYDUREON BCISE immediately after the autoinjector is prepared as a subcutaneous injection in the abdomen, thigh, or upper arm region. Advise patients to use a different injection site each week when injecting in the same region.
- Do not administer BYDUREON BCISE intravenously or intramuscularly.
- Refer patients to the accompanying Instructions for Use for complete administration instructions with illustrations [see Instructions for Use].
2.4 Initiating BYDUREON BCISE Therapy
Prior treatment with an immediate- or extended-release exenatide product is not required when initiating BYDUREON BCISE therapy. Discontinue an immediate- or extended-release exenatide product prior to initiation of BYDUREON BCISE.
Patients changing from immediate-release exenatide to BYDUREON BCISE may experience transient (approximately 2 to 4 weeks) elevations in blood glucose concentrations.
Patients changing from another extended-release exenatide product to BYDUREON BCISE may do so at the next regularly scheduled dose.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
BYDUREON BCISE is contraindicated in patients with:
- A personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- A prior serious hypersensitivity reaction to exenatide or to any of the components of BYDUREON BCISE. Serious hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with exenatide [see Warnings and Precautions (5.7)].
- A history of drug-induced immune-mediated thrombocytopenia from exenatide products. Serious bleeding, which may be fatal, from drug-induced immune-mediated thrombocytopenia has been reported with exenatide use [see Warnings and Precautions (5.8)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Thyroid C-cell Tumors
In both genders of rats, exenatide extended-release caused a dose-related and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and/or carcinomas) at clinically relevant exposures compared to controls [see Nonclinical Toxicology (13.1)]. A statistically significant increase in malignant thyroid C-cell carcinomas was observed in female rats receiving exenatide extended-release at 27-times clinical exposure compared to controls and higher incidences were noted in males above controls in all treated groups at ≥2-times clinical exposure. The potential of exenatide extended-release to induce C-cell tumors in mice has not been evaluated. Other GLP-1 receptor agonists have also induced thyroid C-cell adenomas and carcinomas in male and female mice and rats at clinically relevant exposures. It is unknown whether BYDUREON BCISE will cause thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of exenatide extended-release-induced rodent thyroid C-cell tumors has not been determined.
Cases of MTC in patients treated with liraglutide, another GLP-1 receptor agonist, have been reported in the postmarketing period; the data in these reports are insufficient to establish or exclude a causal relationship between MTC and GLP-1 receptor agonist use in humans.
BYDUREON BCISE is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2. Counsel patients regarding the potential risk of MTC with the use of BYDUREON BCISE and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness).
Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with BYDUREON BCISE. Such monitoring may increase the risk of unnecessary procedures, due to the low specificity of serum calcitonin testing for MTC and a high background incidence of thyroid disease. Significantly elevated serum calcitonin may indicate MTC and patients with MTC usually have values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
5.2 Acute Pancreatitis
Based on postmarketing data, exenatide has been associated with acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis. After initiation of BYDUREON BCISE, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting). If pancreatitis is suspected, BYDUREON BCISE should promptly be discontinued and appropriate management should be initiated. If pancreatitis is confirmed, BYDUREON BCISE should not be restarted. Consider antidiabetic therapies other than BYDUREON BCISE in patients with a history of pancreatitis. In clinical trials of BYDUREON BCISE acute pancreatitis occurred in 0.4% of patients.
5.3 Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
The risk of hypoglycemia is increased when BYDUREON BCISE is used in combination with insulin secretagogues (e.g., sulfonylureas) or insulin. Patients may require a lower dose of the secretagogue or insulin to reduce the risk of hypoglycemia in this setting [see Adverse Reactions (6.1)].
5.4 Acute Kidney Injury
BYDUREON BCISE may induce nausea and vomiting with transient hypovolemia and may worsen renal function. There have been postmarketing reports of altered renal function with exenatide, including increased serum creatinine, renal impairment, worsened chronic renal failure and acute renal failure, sometimes requiring hemodialysis or kidney transplantation. Some of these events occurred in patients receiving one or more pharmacologic agents known to affect renal function or hydration status such as angiotensin converting enzyme inhibitors, nonsteroidal anti-inflammatory drugs, or diuretics. Some events occurred in patients who had been experiencing nausea, vomiting or diarrhea, with or without dehydration. Reversibility of altered renal function has been observed in many cases with supportive treatment and discontinuation of potentially causative agents, including BYDUREON (exenatide extended-release for injectable suspension). BYDUREON BCISE is not recommended for use in patients with an eGFR below 45 mL/min/1.73 m2 [see Use in Specific Populations (8.6)].
5.5 Gastrointestinal Disease
Exenatide has not been studied in patients with severe gastrointestinal disease, including gastroparesis. Because exenatide is commonly associated with gastrointestinal adverse reactions, including nausea, vomiting, and diarrhea, the use of BYDUREON BCISE is not recommended in patients with severe gastrointestinal disease.
5.6 Immunogenicity
Patients may develop antibodies to exenatide following treatment with BYDUREON BCISE. Anti-exenatide antibodies were measured in BYDUREON BCISE-treated patients in two comparator-controlled 28-week studies of BYDUREON BCISE. Patients with higher titer antibodies may have an attenuated HbA1c response. If there is worsening glycemic control or failure to achieve targeted glycemic control, consider alternative antidiabetic therapy [see Adverse Reactions (6.2)].
5.7 Hypersensitivity
There have been postmarketing reports of serious hypersensitivity reactions (e.g., anaphylaxis and angioedema) in patients treated with exenatide. If a hypersensitivity reaction occurs, the patient should discontinue BYDUREON BCISE and promptly seek medical advice [see Contraindications (4) and Adverse Reactions (6.3)]. Inform and closely monitor patients with a history of anaphylaxis or angioedema with another GLP‑1 receptor agonist for allergic reactions, because it is unknown whether such patients will be predisposed to anaphylaxis with BYDUREON BCISE.
5.8 Drug-Induced Thrombocytopenia
Serious bleeding, which may be fatal, from drug-induced immune-mediated thrombocytopenia has been reported in the postmarketing setting with exenatide use. Drug-induced thrombocytopenia is an immune-mediated reaction with exenatide-dependent anti-platelet antibodies. In the presence of exenatide, these antibodies cause platelet destruction. If drug-induced thrombocytopenia is suspected, discontinue BYDUREON BCISE immediately and do not re-expose the patient to exenatide. Upon discontinuation, thrombocytopenia can persist due to the prolonged exenatide exposure from BYDUREON BCISE (about 10 weeks) [see Adverse Reactions (6.3)].
5.9 Injection-Site Reactions
There have been postmarketing reports of serious injection-site reactions (e.g., abscess, cellulitis, and necrosis), with or without subcutaneous nodules, with the use of BYDUREON [see Adverse Reactions (6.3)]. Isolated cases required surgical intervention.
5.10 Acute Gallbladder Disease
Acute events of gallbladder disease have been reported in GLP-1 receptor agonist trials. In the EXSCEL trial [see Clinical Studies (14.2)], 1.9% of BYDUREON-treated patients and 1.4% of placebo-treated patients reported an acute event of gallbladder disease, such as cholelithiasis or cholecystitis. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- Risk of Thyroid C-cell Tumors [see Warnings and Precautions (5.1)]
- Acute Pancreatitis [see Warnings and Precautions (5.2)]
- Hypoglycemia [see Warnings and Precautions (5.3)]
- Acute Kidney Injury [see Warnings and Precautions (5.4)]
- Gastrointestinal Disease [see Warnings and Precautions (5.5)]
- Immunogenicity [see Warnings and Precautions (5.6)]
- Hypersensitivity [see Warnings and Precautions (5.7)]
- Drug-Induced Thrombocytopenia [see Warnings and Precautions (5.8)]
- Injection-Site Reactions [see Warnings and Precautions (5.9)]
- Acute Gallbladder Disease [seeWarnings and Precautions (5.10)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in this section are derived from pooled data from the controlled period of the 2 comparator-controlled trials as well as data from the extension phase of one of these trials [see Clinical Studies (14)]. There were 410 patients exposed to BYDUREON BCISE 2 mg for 28 weeks during the controlled phases, and an additional 116 patients exposed to BYDUREON BCISE 2 mg during an uncontrolled extension for an additional 24 weeks. Overall, there were 526 patients exposed to BYDUREON BCISE 2 mg with a mean duration of exposure of 35 weeks in the controlled and extension phases of the two trials. Across the treatment arms in the controlled periods, the mean age of patients was 55 years, 2% were 75 years or older and 59% were male. The population in these studies was 78% White, 15% Black or African American, 5% Asian; 1% American Indian or Alaska Native; <1 % were Native Hawaiian or Pacific Islander; and <1% were other races. This population included 42% of Hispanic or Latino ethnicity. At baseline, the population had diabetes for an average of 8.3 years and had a mean HbA1c of 8.5%. Baseline estimated renal function was normal or mildly impaired (eGFR ≥60 mL/min/1.73 m2) in 93% of the pooled study populations.
Common Adverse Reactions
Table 1 summarizes the adverse reactions with an incidence ≥5% occurring in BYDUREON BCISE-treated patients in the pooled data from the controlled and extension phases, including 10 weeks of follow‑up, of the two comparator-controlled 28-week clinical trials. Adverse reactions were identified based on known adverse reactions associated with BYDUREON.
Table 1: Adverse Reactions Reported in ≥5% of BYDUREON BCISE-Treated Patients from Pooled Clinical Trial Data in Patients with Type 2 Diabetes Mellitus BYDUREON
BCISE
2 mg
N = 526
%
Injection site nodule
10.5
Nausea
8.2
Note: Percentages are based on the number of patients who were randomized and received at least one dose of BYDUREON BCISE.
Nausea was a common adverse reaction associated with initiation of treatment with BYDUREON BCISE and usually decreased over time with continued use. The incidence of nausea and/or vomiting was 2% in the first week of therapy compared to 1% in the 4th week of therapy.
Less Common Adverse Reactions
Adverse reactions that occurred in >2% and <5% of patients receiving BYDUREON BCISE during the controlled and extension phases, including 10 weeks of follow‑up, of the two comparator-controlled 28-week clinical trials include: headache (4.4%), diarrhea (4.0%), vomiting (3.4%), injection site pruritus (3.2%), dizziness (2.5%), injection site erythema (2.3%), constipation (2.1%).
Adverse Reactions Leading to Discontinuation of Therapy
The incidence of discontinuation of therapy due to adverse reactions was 3.9% for BYDUREON BCISE‑treated patients in the two comparator-controlled 28-week trials. The most common classes of adverse reactions leading to discontinuation of therapy for BYDUREON BCISE-treated patients were Gastrointestinal Disorders 2.0% and General Disorders and Administration Site Conditions 1.2%. For BYDUREON BCISE-treated patients, the most frequent adverse reactions leading to discontinuation of therapy within each of these respective classes were diarrhea (0.7%), nausea (0.7%), vomiting (0.5%) and injection-site nodule (0.5%).
Other Adverse Reactions
Hypoglycemia
Table 2 summarizes the incidence of glucose level <54 mg/dL regardless of hypoglycemia clinical symptoms and the incidence of severe hypoglycemia in the two comparator-controlled 28-week trials of BYDUREON BCISE.
Table 2: Incidence (% of Subjects) of Hypoglycemia (glucose <54 mg/dL) and Severe Hypoglycemia in Clinical Trials in Patients with Type 2 Diabetes Mellitus Incidence of Hypoglycemia (glucose <54 mg/dL)
Mono- or Combination Therapy with One or Two OADs Trial (28 weeks)
With Concomitant Sulfonylurea Use
BYDUREON BCISE 2 mg (N=88)
25.0%
Without Concomitant Sulfonylurea Use
BYDUREON BCISE 2 mg (N=141)
2.1%
Add-On to Metformin Trial (28 weeks)
All treated subjects
BYDUREON BCISE 2 mg (N=181)
0.0%
Incidence of Severe Hypoglycemia
Mono- or Combination Therapy with One or Two OADs Trial (28 weeks)
With Concomitant Sulfonylurea Use
BYDUREON BCISE 2 mg (N=88)
2.3%
Without Concomitant Sulfonylurea Use
BYDUREON BCISE 2 mg (N=141)
0.7%
Add-On to Metformin Trial (28 weeks)
All treated subjects
BYDUREON BCISE 2 mg (N=181)
0.0%
Note: N and percentages are based on the number of patients who were randomized and received at least one dose of BYDUREON BCISE.
Severe hypoglycemia was defined as clinical symptoms that were considered to result from hypoglycemia in which the patient required the assistance of another person and associated with recovery after oral carbohydrates, intravenous glucose or glucagon administration if no plasma glucose was available.
Injection-Site Adverse Reactions
In the two comparator-controlled 28-week trials, injection site reactions (including injection site nodule, injection site pruritus, injection site bruising) were observed in 23.9% of patients treated with BYDUREON BCISE. The formation of subcutaneous nodules is consistent with the properties of the microspheres used in BYDUREON BCISE.
Increase in Heart Rate
In clinical trials of BYDUREON BCISE the mean increase from baseline in heart rate was 2.4 beats per minute.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to exenatide in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Anti-exenatide antibodies were measured at prespecified intervals in 393 BYDUREON BCISE-treated patients in the two comparator-controlled studies. In these trials 42.2% of these patients developed low titer antibodies to exenatide and approximately 31.8% of patients developed high titer antibodies at any time during the studies. The percentage of patients with positive antibody titers, in particular high titers, peaked at approximately Weeks 8-16 of dosing and then diminished over time.
Change in HbA1c from baseline in patients with low titer antibodies at the last visit was generally comparable to that observed in antibody-negative patients at the last visit. However, patients with higher titer antibodies may have an attenuated HbA1c response.
Amongst BYDUREON BCISE-treated patients evaluable for antibodies (N=393), the incidence of potentially immunogenic injection site reactions (most commonly injection site nodule) during the 28-week studies was approximately 19.6%. These reactions were less commonly observed in antibody-negative patients (15.7%) and patients with low titer antibodies (16.3%) compared with those with high titer antibodies (27.2%).
A total of 246 patients with antibodies to exenatide in the BYETTA and BYDUREON clinical trials were tested for the presence of cross-reactive antibodies to GLP-1 and/or glucagon. No treatment-emergent cross-reactive antibodies were observed across the range of titers.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of BYDUREON BCISE or other formulations of exenatide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergy/Hypersensitivity: injection-site reactions, generalized pruritus and/or urticaria, macular or papular rash, angioedema; anaphylactic reaction.
Blood and Lymphatic Systems: drug-induced thrombocytopenia [see Warnings and Precautions (5.8)]
Drug Interactions: increased international normalized ratio (INR) sometimes associated with bleeding, with concomitant warfarin [see Drug Interactions (7)].
Gastrointestinal: nausea, vomiting, and/or diarrhea resulting in dehydration; abdominal distension, abdominal pain, eructation, constipation, flatulence, acute pancreatitis, hemorrhagic and necrotizing pancreatitis sometimes resulting in death [see Indications and Usage (1)].
Neurologic: dysgeusia; somnolence
Renal and Urinary Disorders: altered renal function, including increased serum creatinine, renal impairment, worsened chronic renal failure or acute renal failure (sometimes requiring hemodialysis), kidney transplant and kidney transplant dysfunction.
Skin and Subcutaneous Tissue Disorders: alopecia
-
7 DRUG INTERACTIONS
Table 3: Clinically Relevant Interactions Affecting Drugs Co-Administered with BYDUREON BCISE and Other Exenatide-Containing Products
Orally Administered Drugs (e.g., acetaminophen)
Clinical Impact
Exenatide slows gastric emptying. Therefore, BYDUREON BCISE has the potential to reduce the rate of absorption of orally administered drugs.
[see Clinical Pharmacology (12.3)].
Intervention
Use caution when administering oral medications with BYDUREON BCISE where a slower rate of oral absorption may be clinically meaningful.
Warfarin
Clinical Impact
BYDUREON BCISE has not been studied with warfarin. However, in a drug interaction study, BYETTA did not have a significant effect on INR [see Clinical Pharmacology (12.3)]. There have been postmarketing reports for exenatide of increased INR with concomitant use of warfarin, sometimes associated with bleeding [see Adverse Reactions (6.3)].
Intervention
In patients taking warfarin, the INR should be monitored more frequently after initiating BYDUREON BCISE. Once a stable INR has been documented, the INR can be monitored at the intervals usually recommended for patients on warfarin.
Concomitant Use of Insulin Secretagogues or Insulin
Clinical Impact
Exenatide promotes insulin release from pancreatic beta-cells in the presence of elevated glucose concentrations. The risk of hypoglycemia is increased when exenatide is used in combination with insulin secretagogues (e.g., sulfonylureas) or insulin [see Adverse Reactions (6.1)].
Intervention
Patients may require a lower dose of the secretagogue or insulin to reduce the risk of hypoglycemia in this setting.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited data with exenatide, the active ingredient in BYDUREON BCISE, in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriage. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations). Based on animal reproduction studies, there may be risks to the fetus from exposure to BYDUREON BCISE during pregnancy. BYDUREON BCISE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal reproduction studies identified increased adverse fetal and neonatal outcomes from exposure to exenatide extended-release during pregnancy or from exposure to exenatide during pregnancy and lactation, in association with maternal effects. In rats, exenatide extended-release, administered during the period of organogenesis, reduced fetal growth and produced skeletal ossification deficits at doses that approximate clinical exposures at the maximum recommended human dose (MRHD) of 2 mg/week. In mice, exenatide administered during gestation and lactation, caused increased neonatal deaths at doses that approximate clinical exposures at the MRHD (see Data). Based on animal data, advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with an HbA1c >7 and has been reported to be as high as 20-25% in women with HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryofetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Animal Data
Pregnant rats given subcutaneous doses of 0.3, 1, or 3 mg/kg exenatide extended-release every 3 days during organogenesis had systemic exposures 3-, 8-, and 19-times human exposure, respectively, at the MRHD of 2 mg/week BYDUREON BCISE based on plasma exenatide exposure (AUC) comparison. Reduced fetal growth at all doses and skeletal ossification deficits at 1 and 3 mg/kg occurred at doses that decreased maternal food intake and body weight gain.
In studies evaluating reproduction and development in pregnant mice and rabbits, maternal animals were administered exenatide, the active ingredient in BYDUREON BCISE, by subcutaneous injection twice a day. Differences in embryo-fetal developmental toxicity from subcutaneously injected exenatide extended-release and exenatide were not evaluated in mice, rats, or rabbits.
In pregnant mice given 6, 68, 460, or 760 mcg/kg/day exenatide during fetal organogenesis, skeletal variations associated with slowed fetal growth, including changes in number of rib pairs or vertebral ossifications sites, and wavy ribs were observed at 760 mcg/kg/day, a dose that produced maternal toxicity and yielded systemic exposure 200 times the human exposure resulting from the MRHD of BYDUREON BCISE based on AUC comparison.
In pregnant rabbits given 0.2, 2, 22, 156, or 260 mcg/kg/day exenatide during fetal organogenesis, irregular fetal skeletal ossifications were observed at 2 mcg/kg/day, a dose yielding systemic exposure up to 6 times the human exposure from the MRHD of BYDUREON BCISE based on AUC comparison.
In maternal mice given 6, 68, or 760 mcg/kg/day exenatide from gestation day 6 through lactation day 20 (weaning), an increased number of neonatal deaths at 6 mcg/kg/day were observed on postpartum days 2 to 4 in dams given 6 mcg/kg/day, a dose yielding a systemic exposure equivalent to the human exposure from the MRHD of BYDUREON BCISE based on AUC comparison.
8.2 Lactation
Risk Summary
There is no information regarding the presence of exenatide, in human milk, the effects of exenatide on the breastfed infant, or the effects of exenatide on milk production. Exenatide, the active ingredient in BYDUREON BCISE was present in the milk of lactating mice. However, due to species-specific differences in lactation physiology, the clinical relevance of these data is not clear (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for exenatide and any potential adverse effects on the breastfed child from exenatide or from the underlying maternal condition.
Data
In lactating mice subcutaneously injected twice a day with exenatide, the active ingredient in BYDUREON BCISE, the concentration of exenatide in milk was up to 2.5% of the concentration in maternal plasma.
8.4 Pediatric Use
Safety and effectiveness of BYDUREON BCISE have not been established in pediatric patients. BYDUREON BCISE is not recommended for use in pediatric patients.
8.5 Geriatric Use
In two comparator-controlled 28-week trials, BYDUREON BCISE was studied in 74 patients (18.0%) who were at least 65 years old and 10 patients who were at least 75 years old. No meaningful differences in safety and efficacy were observed between these patients and the overall population, but the small sample size for patients ≥75 years old limits conclusions. In a large cardiovascular outcomes trial, BYDUREON was studied in 2959 patients (40.3%) who were at least 65 years old and of those, 605 patients (8.2%) were at least 75 years old. Use caution when initiating BYDUREON BCISE in elderly patients because they are more likely to have decreased renal function.
8.6 Renal Impairment
Pharmacokinetic studies of renally impaired patients receiving BYDUREON BCISE indicate that there is an increase in exposure in moderate and mild renally impaired patients as compared to patients with normal renal function. BYDUREON BCISE may induce nausea and vomiting with transient hypovolemia and may worsen renal function.
Monitor patients with mild renal impairment for adverse reactions that may lead to hypovolemia. BYDUREON BCISE is not recommended for use in patients with eGFR below 45 mL/min/1.73 m2 or end-stage renal disease. If used in patients with renal transplantation, closely monitor for adverse reactions that may lead to hypovolemia [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Effects of overdoses with BYETTA, another formulation of exenatide, included severe nausea, severe vomiting, and rapidly declining blood glucose concentrations, including severe hypoglycemia requiring parenteral glucose administration. In the event of overdose, appropriate supportive treatment should be initiated according to the patient's clinical signs and symptoms.
-
11 DESCRIPTION
BYDUREON BCISE (exenatide extended-release) injectable suspension is a GLP-1 receptor agonist supplied as a sterile suspension of exenatide extended-release microspheres in an oil-based vehicle of medium chain triglycerides (MCT), in a single-dose autoinjector. Redispersion by mixing provides a white to off-white-opaque suspension to be administered by subcutaneous injection. Each autoinjector contains sufficient suspension to deliver 2 mg of exenatide extended-release in a volume of 0.85 mL.
Exenatide is a 39-amino acid synthetic peptide amide with an empirical formula of C184H282N50O60S and a molecular weight of 4186.6 Daltons. The amino acid sequence for exenatide is shown below.
H-His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2
Exenatide is incorporated in an extended-release microsphere formulation containing the 50:50 poly(D,L-lactide-co-glycolide) polymer (37.2 mg per dose) along with sucrose (0.8 mg per dose), suspended in the vehicle, MCT (774.4 mg per dose).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Incretins, such as glucagon-like peptide-1 (GLP-1), enhance glucose-dependent insulin secretion and exhibit other antihyperglycemic actions following their release into the circulation from the gut. Exenatide is a GLP-1 receptor agonist that enhances glucose-dependent insulin secretion by the pancreatic beta-cell, suppresses inappropriately elevated glucagon secretion, and slows gastric emptying.
The amino acid sequence of exenatide partially overlaps that of human GLP-1. Exenatide is a GLP-1 receptor agonist that has been shown to bind and activate the human GLP-1 receptor in vitro. This leads to an increase in both glucose-dependent synthesis of insulin and in vivo secretion of insulin from pancreatic beta-cells, by mechanisms involving cyclic AMP and/or other intracellular signaling pathways. Exenatide promotes insulin release from pancreatic beta-cells in the presence of elevated glucose concentrations.
12.2 Pharmacodynamics
Exenatide improves glycemic control through the actions described below.
Glucose-Dependent Insulin Secretion
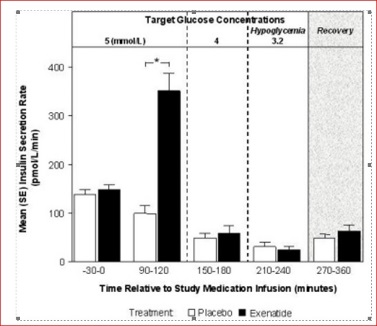
The effect of exenatide infusion on glucose-dependent insulin secretion rates (ISR) was investigated in 11 healthy subjects. In these healthy subjects, on average, the ISR response was glucose-dependent (Figure 1). Exenatide did not impair the normal glucagon response to hypoglycemia.
Figure 1: Mean (SE) Insulin Secretion Rates During Infusion of Exenatide or Placebo by Treatment, Time, and Glycemic Condition in Healthy Subjects
SE = standard error.
Notes: 5 mmol = 90 mg/dL, 4 mmol/L = 72 mg/dL, 3.2 mmol/L = 58 mg/dL; Study medication infusion was started at time = 0 minutes.
Statistical assessments were for the last 30 minutes of each glycemic step, during which the target glucose concentrations were maintained.
*p <0.05, exenatide treatment relative to placebo.Glucagon Secretion
In patients with type 2 diabetes, exenatide moderates glucagon secretion and lowers serum glucagon concentrations during periods of hyperglycemia.
Gastric Emptying
Exenatide slows gastric emptying, thereby reducing the rate at which postprandial glucose appears in the circulation.
Fasting and Postprandial Glucose
In a 12-week clinical pharmacology study of exenatide microspheres suspended in MCT-oil in adults with type 2 diabetes mellitus, reductions in fasting plasma glucose were evident after 2 weeks of treatment, and after 12 weeks resulted in a reduction of fasting plasma glucose concentrations of -40.4 mg/dL, when compared to placebo.
In a clinical study of BYDUREON BCISE, 2‑hour postprandial glucose levels were measured at Week 16, during a mixed meal tolerance test, in a subset of patients with type 2 diabetes mellitus. The mean change from baseline was −78 mg/dL.
Cardiac Electrophysiology
The effect of exenatide at therapeutic (253 pg/mL) and supratherapeutic (627 pg/mL) concentrations, following an intravenous infusion on QTc interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg) three-period crossover thorough QT study in 74 healthy subjects. The upper bound of the one-sided 95% confidence interval for the largest placebo adjusted, baseline-corrected QTc based on population correction method (QTcP) was below 10 ms. Therefore, exenatide was not associated with prolongation of the QTc interval at therapeutic and supratherapeutic concentrations.
12.3 Pharmacokinetics
Absorption
Following a single subcutaneous dose of exenatide microspheres suspended in MCT-oil, there is an initial period of release of surface-bound exenatide followed by a gradual release of exenatide from the microspheres, which results in a peak of plasma exenatide concentration at around Week 6 to Week 7 representing the hydration and erosion of the microspheres.
Following initiation of once every 7 days (weekly) administration of 2 mg BYDUREON BCISE, a gradual increase in the plasma exenatide concentration is observed up to approximately Week 10. From Week 10 mean plasma exenatide concentrations of approximately 208 pg/mL were maintained over once every 7 days (weekly) dosing intervals indicating that steady state was achieved.
Distribution
The mean apparent volume of distribution of exenatide following subcutaneous administration of a single-dose of BYETTA is 28.3 L and is expected to remain unchanged for BYDUREON BCISE.
Metabolism
Elimination
Nonclinical studies have shown that exenatide is predominantly eliminated by glomerular filtration with subsequent proteolytic degradation. The mean apparent clearance of exenatide in humans is 9.1 L/hour and is independent of the dose. Approximately 10 weeks after discontinuation of BYDUREON BCISE therapy, plasma exenatide concentrations generally fall below the minimal quantifiable concentration of 20 pg/mL.
Drug Interaction Studies
The following drug interactions have been studied using BYDUREON. The potential for drug-drug interaction with BYDUREON BCISE is expected to be similar to that of BYDUREON.Acetaminophen
When 1000 mg acetaminophen tablets were administered, either with or without a meal, following 14 weeks of BYDUREON therapy (2 mg weekly), no significant changes in acetaminophen AUC were observed compared to the control period. Acetaminophen Cmax decreased by 16% (fasting) and 5% (fed) and Tmax was increased from approximately 1 hour in the control period to 1.4 hours (fasting) and 1.3 hours (fed).
The following drug interactions have been studied using BYETTA. The potential for drug-drug interaction with BYDUREON BCISE is expected to be similar to that of BYETTA.
Digoxin
Administration of repeated doses of BYETTA 30 minutes before oral digoxin (0.25 mg once daily) decreased the Cmax of digoxin by 17% and delayed the Tmax of digoxin by approximately 2.5 hours; however, the overall steady-state pharmacokinetic exposure (e.g., AUC) of digoxin was not changed.
Lovastatin
Administration of BYETTA (10 mcg twice daily) 30 minutes before a single oral dose of lovastatin (40 mg) decreased the AUC and Cmax of lovastatin by approximately 40% and 28%, respectively, and delayed the Tmax by about 4 hours compared with lovastatin administered alone. In the 30-week controlled clinical trials of BYETTA, the use of BYETTA in patients already receiving HMG CoA reductase inhibitors was not associated with consistent changes in lipid profiles compared to baseline.
Lisinopril
In patients with mild to moderate hypertension stabilized on lisinopril (5-20 mg/day), BYETTA (10 mcg twice daily) did not alter steady-state Cmax or AUC of lisinopril. Lisinopril steady-state Tmax was delayed by 2 hours. There were no changes in 24-hour mean systolic and diastolic blood pressure.
Oral Contraceptives
The effect of BYETTA (10 mcg twice daily) on single and on multiple doses of a combination oral contraceptive (30 mcg ethinyl estradiol plus 150 mcg levonorgestrel) was studied in healthy female subjects. Repeated daily doses of the oral contraceptive (OC) given 30 minutes after BYETTA administration decreased the Cmax of ethinyl estradiol and levonorgestrel by 45% and 27%, respectively, and delayed the Tmax of ethinyl estradiol and levonorgestrel by 3.0 hours and 3.5 hours, respectively, as compared to the oral contraceptive administered alone. Administration of repeated daily doses of the OC one hour prior to BYETTA administration decreased the mean Cmax of ethinyl estradiol by 15%, but the mean Cmax of levonorgestrel was not significantly changed as compared to when the OC was given alone. BYETTA did not alter the mean trough concentrations of levonorgestrel after repeated daily dosing of the oral contraceptive for both regimens. However, the mean trough concentration of ethinyl estradiol was increased by 20% when the OC was administered 30 minutes after BYETTA administration injection as compared to when the OC was given alone. The effect of BYETTA on OC pharmacokinetics is confounded by the possible food effect on OC in this study [see Drug Interactions (7)].
Warfarin
Administration of warfarin (25 mg) 35 minutes after repeated doses of BYETTA (5 mcg twice daily on days 1-2 and 10 mcg twice daily on days 3-9) in healthy volunteers delayed warfarin Tmax by approximately 2 hours. No clinically relevant effects on Cmax or AUC of S- and R-enantiomers of warfarin were observed. BYETTA did not significantly alter the pharmacodynamic properties (e.g., international normalized ratio) of warfarin [see Drug Interactions (7)].
Specific Populations
Patients with Renal Impairment
BYDUREON BCISE has not been studied in patients with severe renal impairment (CrCL <30 mL/min, eGFR <30 mL/min/1.73m2) or end-stage renal disease receiving dialysis. Pharmacokinetic analysis of patients receiving 2 mg BYDUREON BCISE indicated that there was an 28% and 69% higher systemic exposure to exenatide in patients with mild (N=96) or moderate (N=24) renal impairment, respectively, as compared to patients with normal renal function (N=70) [see Warnings and Precautions (5.4) and Use in Specific Populations (8.6)]. In a study of BYETTA in subjects with end-stage renal disease receiving dialysis, mean exenatide exposure increased by 3.4-fold compared to that of subjects with normal renal function [see Warnings and Precautions (5.4) and Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
BYDUREON BCISE has not been studied in patients with acute or chronic hepatic impairment.
Age, Male and Female Patients, Race, and Body Weight
Age, gender, race and body weight did not alter the pharmacokinetics of BYDUREON BCISE in population pharmacokinetic analyses.
Pediatric Patients
BYDUREON BCISE has not been studied in pediatric patients [see Use in Specific Populations (8.4)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Thyroid C-cell tumors have been observed in rats and mice with GLP-1 receptor agonists.
A 2‑year carcinogenicity study was conducted with exenatide extended-release, the active component of BYDUREON BCISE, in male and female rats at doses of 0.3, 1.0, and 3.0 mg/kg (2-, 10-, and 27-times human systemic exposure at the maximum recommended human dose (MRHD) of 2 mg/week. BYDUREON BCISE based on plasma exenatide AUC, respectively) administered by subcutaneous injection every other week. In this study there was an increased incidence of C-cell adenomas and C-cell carcinomas at all doses. An increase in benign fibromas was seen in the skin subcutis at injection sites of males given 3 mg/kg. No treatment-related injection-site fibrosarcomas were observed at any dose. The human relevance of these findings is currently unknown.
Carcinogenicity of exenatide extended-release has not been evaluated in mice.
Exenatide, the active ingredient in BYDUREON BCISE, was not mutagenic or clastogenic, with or without metabolic activation, in the Ames bacterial mutagenicity assay or chromosomal aberration assay in Chinese hamster ovary cells. Exenatide was negative in the in vivo mouse micronucleus assay.
In mouse fertility studies with exenatide, the active ingredient in BYDUREON BCISE, at twice-daily subcutaneous doses of 6, 68, or 760 mcg/kg/day, males were treated for 4 weeks prior to and throughout mating, and females were treated 2 weeks prior to mating and throughout mating until gestation day 7. No adverse effect on fertility was observed at 760 mcg/kg/day, a systemic exposure 163-times the human exposure resulting from the recommended dose of 2 mg/week, based on AUC.
-
14 CLINICAL STUDIES
14.1 Glycemic Control Trials in Adults with Type 2 Diabetes Mellitus
BYDUREON BCISE has been studied as monotherapy and in combination with metformin, a sulfonylurea, a thiazolidinedione, a combination of metformin and a sulfonylurea, or a combination of metformin and a thiazolidinedione.
BYDUREON BCISE versus BYETTA, Both as Monotherapy or as Add-on to Metformin, a Sulfonylurea, a Thiazolidinedione, or Combination of Oral Agents
A 28-week, randomized, open-label comparator-controlled trial was conducted to compare the safety and efficacy of BYDUREON BCISE to BYETTA in patients with type 2 diabetes and inadequate glycemic control with diet and exercise alone or with oral antidiabetic therapy, including metformin, a sulfonylurea, a thiazolidinedione, or a combination of any two of these therapies (NCT01652716).
A total of 375 patients were studied: 278 (74%) were Caucasian, 61(16%) Black or African American, 25 (7%) Asian, 5 (1%) listed as other, 5 (1%) American Indian or Alaska Native, and 1 (<1%) Native Hawaiian or Other Pacific Islander. Patients were treated with diet and exercise alone (13%), a single oral antidiabetic agent (49%), or combination therapy of oral antidiabetic agents (38%). The mean baseline HbA1c was 8.5%. Patients were randomly assigned to receive BYDUREON BCISE 2 mg once every 7 days (weekly) (n=229) or BYETTA (10 mcg twice daily) (n=146), in addition to existing oral antidiabetic agents. Patients assigned to BYETTA initiated treatment with 5 mcg twice daily then increased the dose to 10 mcg twice daily after 4 weeks.
The primary endpoint was change in HbA1c from baseline to Week 28. The results for the primary endpoint at Week 28 are summarized in Table 4. Treatment with BYDUREON BCISE 2 mg once weekly (QW) resulted in a statistically significantly greater reduction in HbA1c compared to BYETTA 10 mcg twice daily. The mean reduction in HbA1c was non-inferior compared with BYETTA 10 mcg twice daily at the pre-specified non-inferiority margin +0.4% in this study. BYDUREON BCISE 2 mg QW was statistically superior to BYETTA 10 mcg twice daily (ANCOVA p-value=0.0032).
Table 4: Results of 28-Week Trial of BYDUREON BCISE versus BYETTA, Both as Monotherapy or as Add-On to Metformin, a Sulfonylurea, a Thiazolidinedione, or Combination of Oral Agents in Patients with Type 2 Diabetes Mellitus BYDUREON BCISE
2 mg QWBYETTA
10 mcg twice daily*- * Least squares means were obtained using an Analysis of Covariance (ANCOVA) model with treatment, baseline HbA1c, baseline HbA1c stratum (<9% or ≥9%), diabetes management method at screening (diet/exercise alone, SU use, or non-SU use), and renal function (normal, mild, or moderate renal impairment) in the population included subjects discontinued treatment before 28 weeks regardless of initiation of rescue medicine.
- † p-value <0.01
Intent-to-Treat Population (N)
229
146
HbA1c (%)
Mean Baseline
8.5
8.5
Mean Change at Week 28
‑1.39
‑1.03
Difference from BYETTA* [95% CI]
‑0.36†
(‑0.66, ‑0.14)
N = number of patients in each treatment group, CI = unadjusted confidence interval, QW = once weekly.
The proportions of subjects achieving HbA1c <7.0% at Week 28 were 40% in BYDUREON BCISE group compared to 38% in BYETTA group. Subjects with missing values at Week 28 counted as non-responders. The mean changes from baseline to Week 28 for fasting plasma glucose were ‑36 mg/dL and ‑27 mg/dL for BYDUREON BCISE and BYETTA, respectively, and for body weight were -1.4 kg and ‑1.9 kg for BYDUREON BCISE and BYETTA, respectively.
BYDUREON BCISE versus Sitagliptin and Placebo, All as Add-on to Metformin Therapy
A 28-week open-label (oral medication blinded), comparator- and placebo-controlled trial was conducted to compare the safety and efficacy of BYDUREON BCISE to sitagliptin and placebo in patients with type 2 diabetes whose glycemic control was inadequate with metformin therapy (NCT01652729).
A total of 364 patients were studied, 296 (81%) were Caucasian, 49 (14%) Black or African American, 14 (4%) Asian and 3 (<1%) American Indian or Alaska Native, 1 (<1%) Native Hawaiian or Other Pacific Islander, and 1 (<1%) was classified otherwise. The mean baseline HbA1c was 8.5%. Patients were randomly assigned to receive BYDUREON BCISE 2 mg once every 7 days (weekly) (n=181), sitagliptin 100 mg/day (n=122) or placebo (n=61), in addition to their existing metformin therapy.
The primary endpoint was change in HbA1c from baseline to Week 28. Results for the primary endpoint at 28 weeks are summarized in Table 5. In this study, treatment with BYDUREON BCISE 2 mg once weekly resulted in a statistically significant mean reduction in HbA1c compared to placebo. BYDUREON BCISE 2 mg was statistically superior to placebo (ANCOVA p-value=0.02).
Table 5: Results of 28-Week Trial of BYDUREON BCISE versus Sitagliptin and Placebo, All as Add-On to Metformin Therapy BYDUREON BCISE
2 mg QWSitagliptin
100 mg/dayPlacebo
once daily- * Least squares means were obtained using an Analysis of Covariance (ANCOVA) model with treatment, baseline HbA1c and baseline HbA1c stratum (<9% or ≥ 9%) in the population included subjects discontinued treatment before 28 weeks regardless of initiation of rescue medicine.
- † Sitagliptin 100 mg/day did not show the superiority to placebo in this study.
- ‡ p-value <0.05
Intent-to-Treat Population (N)
181
122
61
HbA1c (%)
Mean Baseline
8.4
8.5
8.5
Mean Change at Week 28
‑1.07
‑0.79
‑0.59
‑0.28
(‑0.62, 0.02)
Difference from placebo*
[95% CI]‑0.49
(‑0.91, ‑0.07)‡
N = number of patients in each treatment group, CI = unadjusted confidence interval, QW = once weekly.
The proportions of subjects who achieved an HbA1c <7.0% at Week 28 were 41%, 31%, and 26% in BYDUREON BCISE, Sitagliptin and Placebo groups, respectively. Subjects with missing values at Week 28 counted as non-responders. The mean changes from baseline to Week 28 for fasting plasma glucose were ‑24 mg/dL, ‑19 mg/dL and ‑1 mg/dL for BYDUREON BCISE, Sitagliptin and Placebo, respectively, and for body weight were ‑1.4 kg, ‑1.2 kg, and 0.4 kg for BYDUREON BCISE, Sitagliptin and Placebo, respectively.
BYDUREON, another formulation of exenatide extended-release, has been studied as monotherapy and in combination with metformin, sulfonylurea, thiazolidinedione, SGLT2 inhibitor and basal insulin.
BYDUREON Monotherapy versus Metformin, Sitagliptin, and Pioglitazone
A 26-week, randomized, comparator-controlled trial was conducted to compare the safety and efficacy of BYDUREON to metformin, sitagliptin, and pioglitazone in patients with type 2 diabetes whose glycemic control was inadequate with diet and exercise (NCT00676338).
A total of 820 patients were studied: 552 (67%) were Caucasian, 102 (12%) were East Asian, 71 (9%) were West Asian, 65 (8%) were Hispanic, 25 (3.0%) were Black, 4 (0.5%) were Native American, and 1 was classified otherwise. The mean baseline HbA1c was 8.5%. Patients were randomly assigned to receive BYDUREON 2 mg once every seven days (weekly), titrated metformin from 1000 to 2500 mg/day, sitagliptin 100 mg/day or titrated pioglitazone from 30 to 45 mg/day, all dosed according to approved labeling.
The primary endpoint was change in HbA1c from baseline to Week 26 (or the last value at time of early discontinuation). Treatment with BYDUREON 2 mg once weekly (QW) resulted in mean HbA1c reduction that was statistically significantly greater compared to sitagliptin 100 mg/day. The mean reduction in HbA1c was non-inferior compared with metformin 1000-2500 mg/day (mean dose 2077 mg/day at study endpoint). Non-inferiority of BYDUREON 2 mg QW to pioglitazone 30‑45 mg/day (mean dose 40 mg/day at study endpoint) in reducing HbA1c after 26 weeks of treatment was not demonstrated (the mean change from baseline in HbA1c after 26 weeks was ‑1.6% with BYDUREON and ‑1.7% with pioglitazone). The non-inferiority margin was set at +0.3% in this study. The results for the primary endpoint at 26 weeks are summarized in Table 6.
Table 6: Results of 26-Week Trial of BYDUREON Monotherapy versus Metformin, Sitagliptin, and Pioglitazone in Patients with Type 2 Diabetes Mellitus BYDUREON
2 mg QWMetformin
1000-2500
(mean dose
2077) mg/daySitagliptin
100 mg/dayPioglitazone
30-45 (mean
dose 40)
mg/day- * Least squares means were obtained using a mixed model repeated measure analysis with treatment, pooled country, visit, baseline HbA1c value, and treatment by visit interaction as fixed effects, and subject as a random effect.
- † p<0.001, treatment vs comparator.
Intent-to-Treat Population (N)
248
246
163
163
HbA1c (%)
Mean Baseline
8.4
8.6
8.4
8.5
Mean Change at Week 26*
−1.6
−1.5
−1.2
−1.7
Difference from metformin*
[Bonferroni-adjusted 98.3% CI]−0.05
[−0.26, 0.17]Difference from sitagliptin*
[Bonferroni-adjusted 98.3% CI]−0.39†
[−0.63, −0.16]Difference from pioglitazone*
[Bonferroni-adjusted 98.3% CI]0.16
[−0.08, 0.41]N = number of patients in each treatment group.
Note: mean change is least squares mean change.
Note: The primary efficacy analysis was adjusted for multiple comparisons and a two-sided 98.3% confidence interval was utilized to assess difference between treatments.
Note: HbA1c change data at 26 weeks were available from 86%, 87%, 85%, and 82% of the randomized subjects in the BYDUREON, metformin, sitagliptin, and pioglitazone groups, respectively.
QW = once weekly.
The proportion of patients with a Week 26 value achieving HbA1c of less than 7% at Week 26 were 56%, 52%, 40%, and 55% for BYDUREON, metformin, sitagliptin, and pioglitazone, respectively. Patients who did achieve and HbA1c goal <7% and discontinued before Week 26 were not included as responders. The mean changes from baseline to Week 26 for fasting serum glucose were ‑41 mg/dL, ‑36 mg/dL, ‑20 mg/dL and ‑46 mg/dL, and for body weight were ‑2.0 kg, ‑2.0 kg, ‑0.8 kg and +1.5 kg for BYDUREON, metformin, sitagliptin, and pioglitazone, respectively.
BYDUREON versus Sitagliptin and Pioglitazone, All as Add-on to Metformin Therapy
A 26-week double-blind comparator-controlled trial was conducted to compare the safety and efficacy of BYDUREON to sitagliptin and pioglitazone in patients with type 2 diabetes whose glycemic control was inadequate with metformin therapy (NCT00637273).
A total of 491 patients were studied 168 (34.2%) were Caucasian, 143 (29.1%) were Hispanic, 119 (24.2%) were Asian, 52 (10.6%) were Black, 3 (0.6%) were Native American, and 6 (1.2%) were classified otherwise. The mean baseline HbA1c was 8.5%. Patients were randomly assigned to receive BYDUREON 2 mg once every 7 days (weekly), sitagliptin 100 mg/day or pioglitazone 45 mg/day, in addition to their existing metformin therapy.
The primary endpoint was change in HbA1c from baseline to Week 26 (or the last value at time of early discontinuation). In this study, treatment with BYDUREON 2 mg QW resulted in a statistically significant mean HbA1c reduction compared to sitagliptin 100 mg/day. There was a numerically greater reduction in HbA1c with BYDUREON compared to pioglitazone, but there was not sufficient evidence to conclude superiority of BYDUREON 2 mg QW to pioglitazone 45 mg/day in reducing HbA1c after 26 Weeks of treatment. Results for the primary endpoint at 26 Weeks are summarized in Table 7.
Table 7: Results of 26-Week Trial of BYDUREON versus Sitagliptin and Pioglitazone, All as Add-On to Metformin Therapy in Patients with Type 2 Diabetes Mellitus BYDUREON
2 mg QWSitagliptin
100 mg/dayPioglitazone
45 mg/day- * Least squares means were obtained using an ANCOVA model with treatment, baseline HbA1c stratum, and country as fixed effects. Missing Week 26 data (28%, 18%, and 24% for the BYDUREON, sitagliptin, and pioglitazone groups, respectively) were imputed by the LOCF technique.
Intent-to-Treat Population (N)
160
166
165
HbA1c (%)
Mean Baseline
8.6
8.5
8.5
Mean Change at Week 26*
−1.5
−0.9
−1.2
Difference from sitagliptin*
[95% CI]−0.63
[−0.89, −0.37]Difference from pioglitazone*
[95% CI]−0.32
[−0.57, −0.06]N = number of patients in each treatment group.
Note: mean change is least squares mean change.
QW = once weekly.
The proportion of patients with a week 26 value achieving HbA1c of less than 7% at Week 26 were 46%, 30%, and 39% for BYDUREON, sitagliptin, and pioglitazone, respectively. Patients who did achieve an HbA1c goal <7% and discontinued before Week 26 were not included as responders. The mean changes from baseline to Week 26 for fasting serum glucose were ‑32 mg/dL, ‑16 mg/dL and ‑27 mg/dL, and for body weight were ‑2.3 kg, ‑0.8 kg and +2.8 kg for BYDUREON, sitagliptin, and pioglitazone, respectively.
BYDUREON versus Insulin Glargine, Both as Add-on to Metformin or Metformin + Sulfonylurea Therapy
A 26-week open-label comparator-controlled trial was conducted to compare the safety and efficacy of BYDUREON to titrated insulin glargine in patients with type 2 diabetes whose glycemic control was inadequate with metformin or metformin plus sulfonylurea therapy (NCT00641056).
A total of 456 patients were studied: 379 (83.1%) were Caucasian, 47 (10.3%) were Hispanic, 25 (5.5%) were East Asian, 3 (0.7%) were Black, and 2 (0.4%) were West Asian. Background therapy was either metformin (70%) or metformin plus sulfonylurea (30%). The mean baseline HbA1c was 8.3%. Patients were randomly assigned to receive BYDUREON 2 mg once every 7 days (weekly) or insulin glargine once daily in addition to their existing oral antidiabetic therapy. Insulin glargine was dosed to a target fasting glucose concentration of 72 to 100 mg/dL. The mean dose of insulin glargine was 10 units/day at baseline and 31 units/day at endpoint. At Week 26, 21% of insulin glargine treated patients were at fasting glucose goal.
The primary endpoint was change in HbA1c from baseline to Week 26 (or the last value at time of early discontinuation). Treatment with BYDUREON once weekly resulted in a mean reduction in HbA1c from baseline at 26 weeks of -1.5%. The mean reduction in HbA1c seen in insulin glargine arm at 26 weeks was ‑1.3%. The difference in observed effect size between BYDUREON and glargine in this trial excluded the pre-specified non-inferiority margin of +0.3%.
The proportion of patients with a Week 26 value achieving HbA1c of less than 7% at Week 26 were 57% and 48% for BYDUREON and insulin glargine, respectively. Patients who did achieve an HbA1c goal <7% and discontinued before Week 26 were not included as responders. The mean changes from baseline to Week 26 for fasting serum glucose in this study were ‑38 mg/dL and ‑50 mg/dL, and for body weight were ‑2.6 kg and +1.4 kg for BYDUREON and insulin glargine, respectively.
BYDUREON versus Liraglutide, Both as Add-on to Metformin, a Sulfonylurea, Metformin + Sulfonylurea, or Metformin + Pioglitazone Therapy
A 26-week open-label comparator-controlled trial was conducted to compare the safety and efficacy of BYDUREON to liraglutide in patients with type 2 diabetes whose glycemic control was inadequate with metformin, a sulfonylurea, metformin plus sulfonylurea, or metformin plus pioglitazone therapy (NCT01029886).
A total of 911 patients were studied: 753 (82.7%) were Caucasian, 111 (12.2%) were Asian, 32 (3.5%) were American Indian or Alaska Native, 8 (0.9%) were Black, 6 (0.7%) were multiple races, and 1 (0.1%) was Pacific Islander. Background therapy was either a single oral antidiabetic agent (35%) or a combination of oral antidiabetic agents (65%). The mean baseline HbA1c was 8.4%. Patients were randomly assigned to receive BYDUREON 2 mg once every 7 days (weekly) or liraglutide uptitrated from 0.6 mg/day to 1.2 mg/day, then 1.8 mg/day in addition to their existing oral antidiabetic therapy. Each titration was to be completed after at least one week, but could be delayed if the patient had severe nausea or vomiting as established by the investigator. Patients not tolerating the 1.8 mg/day dose of liraglutide by Week 4 were discontinued from the study.
The primary endpoint was change in HbA1c from baseline to Week 26 (or the last value at time of early discontinuation). Treatment with BYDUREON once weekly resulted in a mean reduction in HbA1c from baseline at 26 weeks of -1.3%. The mean reduction in HbA1c seen in the liraglutide arm at 26 weeks was ‑1.5%. The HbA1c reduction with BYDUREON did not meet predefined non-inferiority criteria compared to liraglutide 1.8 mg/day. The non-inferiority margin was set at +0.25% in this study. Results for the primary endpoint at 26 weeks are summarized in Table 8.
Table 8: Results of 26-Week Trial of BYDUREON versus Liraglutide, Both as Add-On to Metformin, a Sulfonylurea, Metformin + Sulfonylurea, or Metformin + Pioglitazone Therapy in Patients with Type 2 Diabetes Mellitus BYDUREON
2 mg QWLiraglutide
1.8 mg/day- * Least squares means were obtained using a mixed model repeated measure analysis with treatment, country, OAD stratum, baseline HbA1c stratum, visit, baseline HbA1c and treatment by visit interaction as fixed effects, and subject as a random effect.
Intent-to-Treat Population (N)
461
450
HbA1c (%)
Mean Baseline
8.5
8.4
Mean Change at Week 26*
−1.3
−1.5
Difference from liraglutide* [95% CI]
0.2 [0.08, 0.33]
N = number of patients in each treatment group.
Note: mean change is least squares mean change.
Note: HbA1c change data at 26 weeks were available from 85% and 86% of the randomized subjects in the BYDUREON and liraglutide groups, respectively.
QW = once weekly.
The proportion of patients with a Week 26 value achieving HbA1c of less than 7% at Week 26 were 48% and 56% for BYDUREON and liraglutide, respectively. Patients who did achieve an HbA1c goal <7% and discontinued before Week 26 were not included as responders. The mean changes from baseline to week 26 for fasting serum glucose were ‑32 mg/dL and ‑38 mg/dL, and for body weight were -2.7 kg and ‑3.6 kg for BYDUREON and liraglutide, respectively.
BYDUREON in Combination with Dapagliflozin versus BYDUREON Alone and Dapagliflozin Alone, All as Add-On to Metformin
A 28‑week double‑blind comparator controlled trial was conducted to compare the efficacy of BYDUREON and dapagliflozin (an SGLT2 inhibitor) to BYDUREON alone and dapagliflozin alone in patients with type 2 diabetes with inadequate glycemic control with metformin therapy (NCT02229396).
A total of 694 patients were studied; 580 (83.6%) were Caucasian, 96 (13.8%) were Black, 5 (0.7%) were Asian, 2 (0.3%) were American Indian or Alaska Native, and 11 (1.6%) were classified otherwise. The mean baseline HbA1c was 9.3%. All patients entered a 1‑week placebo lead–in period. Patients with HbA1c ≥8.0% and ≤12% and on metformin at a dose of at least 1,500 mg per day were randomly assigned to receive either BYDUREON 2 mg once every 7 days (weekly) plus dapagliflozin 10 mg once daily, BYDUREON 2 mg once weekly, or dapagliflozin 10 mg once daily.
The primary endpoint was change in HbA1c from baseline to Week 28. At Week 28, BYDUREON in combination with dapagliflozin provided statistically significantly greater reductions in HbA1c (‑1.77%) compared to BYDUREON alone (‑1.42%, p=0.012) and dapagliflozin alone (‑1.32%, p=0.001). BYDUREON in combination with dapagliflozin provided statistically significantly greater reductions in FPG (‑57.35 mg/dL) compared to BYDUREON alone (‑40.53, p <0.001) and dapagliflozin alone (‑44.72 mg/dL, p=0.006).
BYDUREON versus Placebo, Both as Add-On to Basal Insulin or Basal Insulin + Metformin Therapy
A 28‑week, double‑blind, placebo‑controlled trial was conducted to compare the safety and efficacy of BYDUREON to placebo when added to basal insulin glargine, with or without metformin, in patients with type 2 diabetes with inadequate glycemic control (NCT02229383).
A total of 460 patients were studied: 400 (87.0%) were White, 47 (10.2%) were Black or African American, 6 (1.3%) were Asian, 1 (0.2%) was American Indian or Alaska Native, 1 (0.2%) was Pacific Islander, and 5 (1.1%) were classified otherwise. Patients on sulfonylurea therapy discontinued sulfonylurea. Patients on metformin continued on the same dose of metformin. All patients initially entered an 8‑week insulin dose‑titration phase. Insulin glargine was to be titrated every 3 days with an aim of achieving a target fasting plasma glucose concentration of 72 to 99 mg/dL. Following the titration period, patients with HbA1c ≥7.0% and ≤10.5% were then randomly assigned to receive either BYDUREON 2 mg once every 7 days (weekly) or placebo once every 7 days (weekly).
The primary endpoint was the change in HbA1c from baseline to Week 28. Compared to placebo, treatment with BYDUREON resulted in a statistically significant reduction in mean HbA1c from baseline to Week 28 (Table 9).
Table 9: Results of 28-Week Trial of BYDUREON versus Placebo, Both as Add-On to Insulin Glargine or Insulin Glargine + Metformin BYDUREON
2 mg QWPlacebo
QW- * Adjusted LS means and treatment group difference(s) in the change from baseline values at Week 28 using a multiple imputation method that models a “wash-out” for patients having missing data who discontinued treatment. ANCOVA was used with treatment, region, baseline HbA1c stratum (<9.0% or ≥9.0%), and baseline SU-use stratum (yes vs. no) as fixed factors, and baseline value as a covariate.
- † p-value <0.001 (adjusted for multiplicity).
- ‡ Categories are derived from continuous measurements. All patients with missing endpoint data are imputed as non-responders. Treatment comparison is based on Cochran-Mantel-Haenszel (CMH) test stratified by baseline HbA1c (<9.0% or ≥9.0%), and baseline SU-use stratum (yes vs. no). P-values are from the general association statistics.
Analyses include measurements post rescue therapy and post premature discontinuation of study medication.Intent-to-Treat Population (N)
231
229
Mean HbA1c (%)
Mean Baseline
8.53
8.53
Mean Change at Week 28*
-0.88 (0.070)
-0.24 (0.069)
Difference from Placebo [95% CI]
-0.64†
[-0.83, -0.45]
Percentage Achieving HbA1c <7.0% at Week 28 (%)‡
32.5†
7.0
N = number of patients in each treatment group, CI = confidence interval, QW = once weekly.
Note: mean change is least squares mean change.
The mean change in fasting plasma glucose from baseline to Week 28 was -12.50 mg/dL for BYDUREON and -2.26 mg/dL for placebo. The mean change from baseline to Week 28 in body weight was -0.92 kg for BYDUREON and +0.38 kg for placebo.
14.2 EXSCEL Cardiovascular Outcomes Trial in Patients with Type 2 Diabetes
EXSCEL was a multinational, placebo-controlled, double-blind, randomized, parallel group pragmatic study that evaluated cardiovascular (CV) outcomes during treatment with BYDUREON (exenatide extended-release for injectable suspension) in patients with type 2 diabetes and any level of CV risk when added to the current usual care (NCT01144338).
A total of 14,752 patients were randomized 1:1 to either BYDUREON 2 mg once weekly or placebo and followed as in routine clinical practice for a median of 38.7 months with a median treatment duration of 27.8 months. Ninety six percent of the patients in both treatment groups completed the study in accordance with the protocol, and the vital status was known at the end of the study for 98.9% and 98.8% of the patients in the BYDUREON and placebo group, respectively. The mean age at study entry was 62 years (21 to 92 years with 8.5% of the patients ≥75 years). Approximately 62.0% of the patients were male, 75.8% were Caucasian, 9.8% were Asian, 6.0% were Black, and 20.5% were Hispanic or Latino. The mean BMI was 32.7 kg/m2 and the mean duration of diabetes was 13.1 years. Approximately 49.3% had mild renal impairment (estimated glomerular filtration rate [eGFR] ≥60 to ≤89 mL/min/1.73 m2) and 21.6% had moderate renal impairment (eGFR ≥30 to ≤59 mL/min/1.73 m2).
The mean HbA1c was 8.1%. At baseline, 1.5% of patients were not treated with either oral antidiabetic medications or insulin, 42.3% were treated with one oral antidiabetic medication and 42.4% were treated with two or more oral antidiabetic medications. Usage of oral antidiabetic medications included metformin (76.6%), sulfonylurea (36.6%), DPP-4 inhibitors (14.9%), thiazolidinediones (3.9%), and SGLT-2 inhibitors (0.9%). Overall insulin usage was 46.3% (13.8% with insulin alone and 32.6% with insulin and one or more oral antidiabetic medications).
Overall, at baseline, 26.9% of patients did not have established cardiovascular (CV) disease, while 73.1% had established CV disease. The concomitant use of CV medications (e.g., ACE inhibitors, angiotensin receptor blockers, diuretics, beta blockers, calcium channel blockers, antithrombotic and anticoagulants, and lipid-lowering agents) was similar in the BYDUREON and placebo groups. At baseline, the mean systolic blood pressure was 135.5 mmHg, the mean diastolic blood pressure was 78.1 mmHg, the mean LDL was 95.0 mg/dL, and the mean HDL was 44.0 mg/dL.
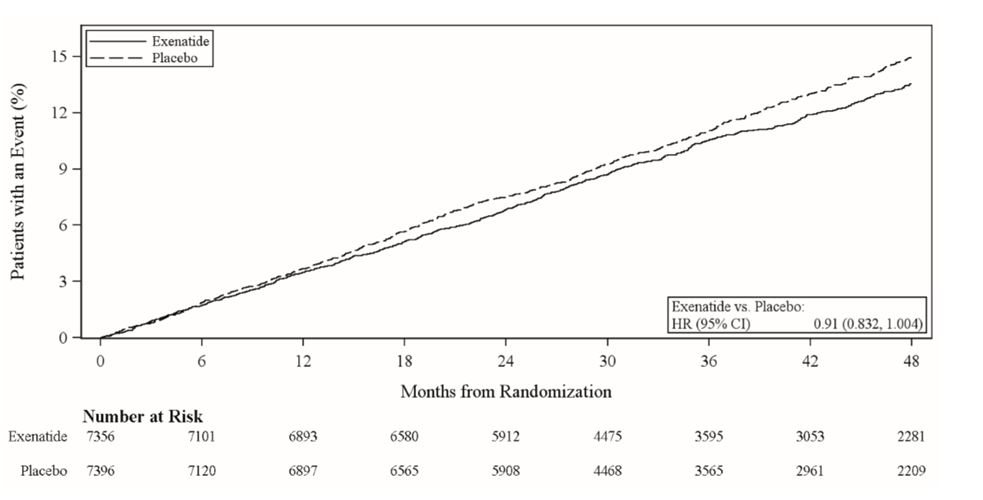
The primary endpoint in EXSCEL was the time to first confirmed Major Adverse Cardiac Event (MACE) from randomization. MACE was defined as occurrence of either a cardiovascular (CV)-related death, or a nonfatal myocardial infarction (MI) or a nonfatal stroke. All-cause mortality, CV-related death, and fatal or nonfatal MI or stroke, hospitalization for acute coronary syndrome, and hospitalization for heart failure were also assessed as secondary endpoints.
A Cox proportional hazards model was used to test for non-inferiority against the pre-specified risk margin of 1.3 for the hazard ratio of MACE and superiority on MACE if non-inferiority was demonstrated. Type-1 error was controlled across multiples tests using a hierarchical testing strategy.
BYDUREON did not increase the risk of MACE in patients with type 2 diabetes mellitus (HR: 0.91; 95% CI: 0.832, 1.004; P<0.001 for non-inferiority; P=0.06 for superiority). See results in Table 10 and Figure 2. The incidence of MACE in patients with and without established CV disease was 13.4% in the BYDUREON group versus 14.6% in the placebo group and 6.0% (BYDUREON) versus 5.9% (placebo), respectively. Five hundred and seven (507) patients (6.9%) died in the BYDUREON group versus 584 (7.9%) in the placebo group.
Table 10: Analysis of Primary Composite Endpoint MACE and Its Components in Patients with Type 2 Diabetes BYDUREON
N=7356Placebo
N=7396HR*
(95% CI)- * HR (active/placebo) and CI are based on Cox proportional hazards regression model, stratified by established CV disease, with treatment group only as explanatory variable.
MACE Composite of CV death, nonfatal MI or nonfatal stroke (time to first confirmed event)
839 (11.4%)
905 (12.2%)
0.91
(0.832, 1.004)
Cardiovascular Death
340 (4.6%)
383 (5.2%)
0.88
(0.76, 1.02)
Nonfatal Myocardial Infarction
466 (6.3%)
480 (6.5%)
0.96
(0.85, 1.09)
Nonfatal Stroke
169 (2.3%)
193 (2.6%)
0.86
(0.70, 1.06)
N=number of patients in each treatment group, HR=hazard ratio, CI=confidence interval, CV=cardiovascular, MI=myocardial infarction.
Figure 2: Time to First Adjudicated MACE in Patients with Type 2 Diabetes
HR=hazard ratio, CI=confidence interval.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
- BYDUREON BCISE contains 2 mg of exenatide in 0.85 mL vehicle, in a pre-filled, single-dose autoinjector. Redispersion by mixing provides a white to off-white, opaque, extended-release injectable suspension, available in cartons that contain four single-dose autoinjectors (NDC: 0310-6540-04).
Storage and Handling
- BYDUREON BCISE must be stored FLAT.
- Store the autoinjector in the original package. Protect from light.
- BYDUREON BCISE should be stored in the refrigerator at 36°F to 46°F (2°C to 8°C), up to the expiration date or until preparing for use. BYDUREON BCISE should not be used past the expiration date. The expiration date can be found on the carton, or on the autoinjector label.
- BYDUREON BCISE can be kept at room temperature not to exceed 86°F (30°C) for no more than a total of 4 weeks, if needed.
- Discard BYDUREON BCISE after use in a puncture-resistant container.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Risk of Thyroid C-cell Tumors
Inform patients that exenatide extended-release causes benign and malignant thyroid C-cell tumors in rats and that the human relevance of this finding has not been determined. Counsel patients to report symptoms of thyroid tumors (e.g., a lump in the neck, hoarseness, dysphagia, or dyspnea) to their physician [see Boxed Warning and Warnings and Precautions (5.1)].
Risk of Pancreatitis
Inform patients treated with BYDUREON BCISE of the potential risk for pancreatitis. Explain that persistent severe abdominal pain that may radiate to the back, and which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to discontinue BYDUREON BCISE promptly and contact their healthcare provider if persistent severe abdominal pain occurs [see Warnings and Precautions (5.2)].
Risk of Hypoglycemia
Inform patients that the risk of hypoglycemia is increased when BYDUREON BCISE is used in combination with an agent that induces hypoglycemia, such as a sulfonylurea or insulin [see Warnings and Precautions (5.3)]. Explain the symptoms, treatment, and conditions that predispose to the development of hypoglycemia. Review and reinforce instructions for hypoglycemia management when initiating BYDUREON BCISE therapy, particularly when concomitantly administered with a sulfonylurea or insulin [see Warnings and Precautions (5.3)].
Risk of Acute Kidney Injury
Inform patients treated with BYDUREON BCISE of the potential risk for worsening kidney function and explain the associated signs and symptoms of renal impairment, as well as the possibility of dialysis as a medical intervention if renal failure occurs [see Warnings and Precautions (5.4)].
Risk of Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions have been reported during postmarketing use of exenatide. Inform patients that if symptoms of hypersensitivity reaction occur, stop taking BYDUREON BCISE and seek medical advice promptly [see Warnings and Precautions (5.7)].
Risk of Drug-Induced Thrombocytopenia
Inform patients that drug-induced immune mediated thrombocytopenia has been reported during use of exenatide. Inform patients that if symptoms of thrombocytopenia occur, e.g. bleeding, stop taking BYDUREON BCISE and seek medical advice promptly [see Warnings and Precautions (5.8)].
Risk of Injection-Site Reactions
Inform patients that there have been postmarketing reports of serious injection-site reactions with or without subcutaneous nodules, with the use of BYDUREON. Isolated cases of injection-site reactions required surgical intervention. Advise patients to seek medical advice if symptomatic nodules occur, or for any signs or symptoms of abscess, cellulitis, or necrosis [see Warnings and Precautions (5.9)].
Acute Gallbladder Disease
Inform patients of the potential risk for cholelithiasis or cholecystitis. Instruct patients to contact their physician if cholelithiasis or cholecystitis is suspected for appropriate clinical follow-up [see Warnings and Precautions (5.10)].
Instructions
Train patients on how to use BYDUREON BCISE properly prior to self-administration. Instruct patients on proper mixing and injection technique to ensure the product is adequately mixed and a full dose is delivered. Refer patients to the accompanying Instructions for Use for complete administration instructions with illustrations.
Inform patients formerly on BYETTA who start BYDUREON BCISE may experience transient elevations in blood glucose concentrations, which generally improve within the first 4 weeks after initiation of therapy [see Dosage and Administration (2.4)].
Treatment with BYDUREON BCISE may also result in nausea, particularly upon initiation of therapy [see Adverse Reactions (6)].
Inform patients about the importance of proper storage of BYDUREON BCISE [see How Supplied/Storage and Handling (16)].
Instruct the patient to review the BYDUREON BCISE Medication Guide and the Instructions for Use each time the prescription is refilled.
Manufactured for:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
By:
Amylin Ohio LLC
West Chester, OH 45071
and
Vetter Pharma-Fertigung GmbH & Co. KG
88214 Ravensburg
Germany
BYDUREON, BCISE and BYETTA are trademarks of the AstraZeneca group of companies.
-
MEDICATION GUIDE
MEDICATION GUIDE
BYDUREON BCISE® (by-DUR-ee-on B-cise)
(exenatide extended-release)
injectable suspension, for subcutaneous useWhat is the most important information I should know about BYDUREON BCISE?
BYDUREON BCISE may cause serious side effects, including:
- Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rats, BYDUREON and medicines that work like BYDUREON caused thyroid tumors, including thyroid cancer. It is not known if BYDUREON BCISE will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
- Do not use BYDUREON BCISE if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC), or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
What is BYDUREON BCISE?
- BYDUREON BCISE is an injectable prescription medicine that may improve blood sugar (glucose) in adults with type 2 diabetes mellitus and should be used along with diet and exercise.
- BYDUREON BCISE is not recommended as the first choice of medicine for treating diabetes.
- BYDUREON BCISE is not a substitute for insulin and is not for use in people with type 1 diabetes or people with diabetic ketoacidosis.
- It is not known if BYDUREON BCISE can be used with mealtime insulin.
- BYDUREON BCISE and BYDUREON are long-acting forms of the medicine in BYETTA (exenatide). BYDUREON BCISE should not be used at the same time as BYETTA or BYDUREON.
- It is not known if BYDUREON BCISE can be used in people who have had pancreatitis.
- It is not known if BYDUREON BCISE is safe and effective for use in children.
Do not use BYDUREON BCISE if:
- you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- you have a history of low blood platelet count from using exenatide medicines (drug-induced thrombocytopenia).
- you are allergic to exenatide or any of the ingredients in BYDUREON BCISE. See the end of this Medication Guide for a complete list of ingredients in BYDUREON BCISE.
Before using BYDUREON BCISE, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had problems with your pancreas or kidneys.
- have severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food.
- are pregnant or plan to become pregnant. BYDUREON BCISE may harm your unborn baby. Tell your healthcare provider if you become pregnant while using BYDUREON BCISE. Talk to your healthcare provider about the best way to control your blood sugar if you plan to become pregnant or while you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if BYDUREON BCISE passes into your breast milk. You should talk with your healthcare provider about the best way to feed your baby while using BYDUREON BCISE.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. BYDUREON BCISE may affect the way some medicines work and some medicines may affect the way BYDUREON BCISE works.
Before using BYDUREON BCISE, talk to your healthcare provider about low blood sugar and how to manage it. Tell your healthcare provider if you are taking other medicines to treat diabetes including insulin or sulfonylureas.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use BYDUREON BCISE?
- Read the Instructions for Use that comes with BYDUREON BCISE.
- Use BYDUREON BCISE exactly as your healthcare provider tells you to.
- BYDUREON BCISE should be injected right away after you prepare your dose.
- Your healthcare provider should show you how to use BYDUREON BCISE before you use it for the first time.
- BYDUREON BCISE is injected under the skin (subcutaneously) of your stomach (abdomen), thigh, or upper arm. Do not inject BYDUREON BCISE into a muscle (intramuscularly) or vein (intravenously).
- Use BYDUREON BCISE 1 time each week on the same day each week at any time of the day.
- BYDUREON BCISE may be taken with or without food.
- If you miss a dose of BYDUREON BCISE, take the missed dose as soon as possible if there are at least 3 days (72 hours) until your next scheduled dose. If there are less than 3 days remaining, skip the missed dose and take your next dose on the regularly scheduled day. Do not take 2 doses of BYDUREON BCISE within 3 days of each other.
- You may change the day of the week as long as your last dose was given 3 or more days before.
- If you use a different long acting exenatide medicine and your healthcare provider switches your medicine to BYDUREON BCISE, you should start using BYDUREON BCISE at your next scheduled dose.
- Do not mix insulin and BYDUREON BCISE together in the same injection.
- You may give an injection of BYDUREON BCISE and insulin in the same body area (such as, your stomach area), but not right next to each other.
- Change (rotate) your injection site with each weekly injection. Do not use the same site for each injection.
- Your dose of other diabetes medicines may need to change because of: change in level of physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, or because of other medicines you take.
- Do not share your BYDUREON BCISE with another person. You may give another person an infection or get an infection from them.
What are the possible side effects of BYDUREON BCISE?
BYDUREON BCISE may cause serious side effects, including:
- See "What is the most important information I should know about BYDUREON BCISE?"
- inflammation of your pancreas (pancreatitis). Stop using BYDUREON BCISE and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
- low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use BYDUREON BCISE with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include:
- o dizziness or light-headedness
- o sweating
- o confusion or drowsiness
- o headache
- o blurred vision
- o slurred speech
- o shakiness
- o fast heartbeat
- o anxiety, irritability, or mood changes
- o hunger
- o weakness
- o feeling jittery
- kidney problems. In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems to get worse or kidney failure.
- stomach problems. Other medicines like BYDUREON BCISE may cause severe stomach problems. It is not known if BYDUREON BCISE causes or worsens stomach problems.
- low blood platelet count (drug-induced thrombocytopenia). BYDUREON BCISE may cause the number of platelets in your blood to be reduced. When your platelet count is too low, your body cannot form blood clots. You could have serious bleeding that could lead to death. Stop using BYDUREON BCISE and call your healthcare provider right away if you have unusual bleeding or bruising. Your blood platelet count may continue to be low for about 10 weeks after stopping BYDUREON BCISE.
- serious allergic reactions. Stop using BYDUREON BCISE and get medical help right away if you have any symptoms of a serious allergic reaction, including itching, rash, or difficulty breathing.
- injection-site reactions. Serious injection-site reactions, with or without bumps (nodules), have happened in some people who use BYDUREON. Some of these injection-site reactions have required surgery. Call your healthcare provider if you have any symptoms of an injection-site reaction, including severe pain, swelling, blisters, an open wound, a dark scab.
- gallbladder problems. Gallbladder problems have happened in some people who take BYDUREON or other medicines like BYDUREON. Tell your healthcare provider right away if you get symptoms of gallbladder problems which may include: pain in the right or middle upper stomach area, nausea and vomiting, fever, or your skin or the white part of your eyes turns yellow.
The most common side effects of BYDUREON BCISE may include a bump (nodule) at the injection site and nausea.
Nausea is most common when you first start using BYDUREON BCISE but decreases over time in most people as their body gets used to the medicine.
Talk to your healthcare provider about any side effect that bothers you or does not go away.
These are not all the possible side effects of BYDUREON BCISE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Keep BYDUREON BCISE and all medicines out of the reach of children.
General information about the safe and effective use of BYDUREON BCISE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BYDUREON BCISE for a condition for which it was not prescribed. Do not give your BYDUREON BCISE to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about BYDUREON BCISE that is written for health professionals.
What are the ingredients in BYDUREON BCISE?
Contents of the powder:
Active Ingredient: exenatide
Inactive Ingredients: polylactide-co-glycolide and sucrose
Contents of liquid (diluent):
Inactive Ingredients: medium chain triglycerides
BYDUREON, BCISE and BYETTA are trademarks of the AstraZeneca group of companies. All other marks are the marks of their respective owners.
Manufactured for:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850By:
Amylin Ohio LLC
West Chester, OH 45071 andVetter Pharma-Fertigung GmbH & Co. KG
88214 Ravensburg
Germany
For more information about BYDUREON BCISE, go to www.BYDUREONBCISE.com or call 1-877-700-7365.
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: February 2020
-
INSTRUCTIONS FOR USE
Once-weekly
BYDUREON BCISE®(exenatide extended-release), injectable suspension
For subcutaneous use only
Single-dose Autoinjector once weekly2 mg
Read the Instructions for Use before you start using BYDUREON BCISE.
Before using BYDUREON BCISE, talk to your healthcare provider about how to use it the right way.Before You Begin
The autoinjector:
- - Is a single use, fixed dose autoinjector that automatically injects your medicine.
- - Is injected 1 time per week under the skin.
- - Comes in the locked position before you use it. Do not unlock the autoinjector until you are ready to inject it.
- - Needle is hidden. You do not see it before, during, or after using the autoinjector.
- Do not use the autoinjector if any parts look to be broken or damaged.
- Store flat in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Never share your BYDUREON BCISE autoinjector with anyone else. You may give an infection to them or get an infection from them.
- BYDUREON BCISE should not be used by people who are blind or cannot see well, unless another person who is trained to use this device can help.
- Keep the autoinjector, and all medicines, out of the reach of children.
Before Use
Supplies needed to give your injection:
BYDUREON BCISE autoinjector, Alcohol swab, A clean, flat surface, Sharps container (see “disposal” instructions at the end of these instructions)
Step 1: Prepare for Injection
A. Let your autoinjector come to room temperature.
Remove 1 autoinjector from the refrigerator and rest it flat for 15 minutes.
Autoinjector can be kept at room temperature for up to 4 weeks.B. Check the expiration date (labeled EXP) printed on the autoinjector label.
Do not use the autoinjector past the expiration date. If the expiration date has passed, throw it away and get a new autoinjector.C. Wash your hands.
D. Choose your injection site.
You can inject into your stomach, thigh, or back of the upper arm, see Figure D.Each week you can use the same area of your body, but choose a different injection site in that area of your body.
Clean the area with an alcohol swab.Step 2: Mix the medicine
A. Look in the window.
You may see white medicine along the sides, bottom or top. This means the medicine is not mixed evenly.B. Shake the autoinjector hard,
in an up-and-down motion, until the medicine is mixed evenly and you do not see any white medicine along the sides, bottom or top. Shake for at least 15 seconds. The autoinjector may need to be shaken longer than 15 seconds if the autoinjector has not been correctly stored flat.C. Check the mix.
Hold the autoinjector up to the light and look through both sides and the bottom of the window. If not mixed well, repeat Step 2 and check again.Do not go to the next step unless your medicine is mixed well. To get a full dose, the medicine must be mixed well and look cloudy.
If not mixed well, continue to shake hard.Step 3: Prepare the Autoinjector
Important: After the medicine is fully mixed, you must complete the preparation steps right away, and inject to get the full dose. Do not save it to use later.
Only unlock the autoinjector when you are ready to inject
A. Unlock the autoinjector.
Hold the autoinjector up straight with the orange cap toward the ceiling. Turn the knob from the Lock to the Unlock position until you hear a click.B. While still holding the autoinjector straight up, firmly unscrew the orange cap.
- You may need to turn the cap a few times before it loosens (if you hear clicking you are turning in the wrong direction).
- Continue holding the autoinjector upright to prevent the medicine from accidently leaking.
- A green shield will pop up after the cap is removed. The green shield hides the needle.
It is normal to see a few drops of liquid inside the cap. Do not recap the autoinjector.
Throw away the cap.Step 4: Inject the Dose
A. Inject and hold:
- Push the autoinjector against your skin. You will hear a “click” when the injection begins.
- Keep holding the autoinjector against the skin for 15 seconds. This is to make sure you get the full dose.
B. Make sure you received your full dose.
After you receive your injection, you will see an orange rod in the window. After you lift the autoinjector from your skin, the green shield will move back up to lock over the needle. See the Common Questions and Answers for what to do if you do not see the orange rod in the window after injection.Step 4: Inject the Dose (continued)
C. Disposal.
Put your used autoinjector in a FDA-cleared sharps disposal container right away after use.
Do not throw away (dispose of) loose needles and syringes into your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- Made of heavy-duty plastic
- Can be closed with a tight-fitting, puncture-resistant lid that will not let sharps come out
- Upright and stable during use
- Leak-resistant, and
- Properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container. See “Common Questions and Answers” for additional disposal information.
Please keep these instructions to use for your next dose.
Common Questions and Answers
1. Where is the needle?
The needle is attached to the autoinjector and covered by the orange cap.
When you unscrew the orange cap, the green shield keeps the needle covered
until you inject.
For more information, please see Figure N in Step 3B in the Instructions for Use.2. How do I know if the medicine is fully mixed?
After shaking the autoinjector, look through both sides of the window. You should not see any white medicine along the bottom, top, or sides. If you see white medicine, it is unmixed. To mix, shake the autoinjector hard until the white medicine is no longer on the bottom, top, or sides. The medicine should look even throughout.3. Why do I need to hold the autoinjector upright while removing the orange cap?
Holding the autoinjector with the orange cap straight up helps prevent the medicine from leaking. It is normal to see a few drops of medicine inside the orange cap after you unscrew it.4. Why should I inject my medicine right away after mixing it?
If you do not inject your medicine right away after mixing, the medicine may separate, and you will not get your full dose. You can re-mix your medicine if your autoinjector is in the locked position. However, after you unlock it, you must complete the preparation steps right away and inject to get the full dose. You cannot save it for later use.5. How do I know I gave myself the full dose of medicine?
To be sure you get your full dose, press and hold the autoinjector against your skin.
You will feel the needle go into your skin. Hold the needle against your skin for 15 seconds. This will allow enough time for all the medicine to go from the autoinjector to under your skin. After removing the needle, look for the orange rod in the window as a way to tell that the dose has been given. If the orange rod does not appear contact Customer Service at 1-877-700-7365.6. Why should I store my autoinjectors flat in the refrigerator?
Autoinjectors stored vertically (with the needle up or down) are more difficult to mix. The medicine can still be fully mixed, but it will take more shaking and more time.7. What if I do not have an FDA-cleared sharps disposal container?
Do not throw away (dispose of) the autoinjector in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:- Made of heavy-duty plastic
- Can be closed with a tight-fitting, puncture-resistant lid, that won’t let sharps come out
- Upright and stable during use
- Leak-resistant
- Properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and autoinjectors.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
8. What if I cannot unlock the autoinjector?
Review the Instructions for Use Step 3 to make sure you are following the right instructions, then contact Customer Service, 1-877-700-7365 for help as needed. Do not try to unlock with excessive force or tools.9. What if I cannot remove the orange cap from the autoinjector?
Review the Instructions for Use Step 3 to make sure you are following the right instructions. You should also check that the knob is fully in the unlocked position, then contact Customer Service, 1-877-700-7365 for help as needed. Do not use tools or try to force the cap off.10. For other questions about BYDUREON BCISE:
Visit www.BydureonBCise.com.
Call Customer Service at 1-877-700-7365.How to Store BYDUREON BCISE Autoinjector
- Store the autoinjector flat in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Each autoinjector can be kept at room temperature not to exceed 86°F (30°C) for no more than a total of 4 weeks, if needed.
- Store in the packaging provided to protect from light until you are ready to prepare and use your dose.
- Do not use the autoinjector past the expiration date. The expiration date is labeled EXP.
- Keep the autoinjector clean and away from spills.
- This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: 7/2019
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL- 2 mg Pen Label
NDC: 0310-6540-01
2 mg single-dose autoinjectorMedication requires mixing before use
Store FLAT in a refrigerator 36-46°F
(2-8°C)
Once-weekly
BYDUREON BCise®
(exenatide extended-release)injectable suspension
For subcutaneous use only
Rx only AstraZeneca
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 2 mg Carton
NDC: 0310-6540-04
Once-WeeklyBYDUREON BCise® 2 mg single-dose autoinjector
(exenatide extended-release)injectable suspension
For subcutaneous use only
Dispense with enclosed Medication Guide
to each patient.
Store FLAT in refrigerator 36-46°F (2-8°C).
Total Quantity: 4 x Single-Dose Autoinjectors
Use one autoinjector every weekEach autoinjector delivers a 2 mg dose
Rx only AstraZeneca
-
INGREDIENTS AND APPEARANCE
BYDUREON BCISE
exenatide injection, suspension, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0310-6540 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EXENATIDE (UNII: 9P1872D4OL) (EXENATIDE - UNII:9P1872D4OL) EXENATIDE 2 mg in 0.85 mL Inactive Ingredients Ingredient Name Strength POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 63000 MW) (UNII: QD923V64H3) SUCROSE (UNII: C151H8M554) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) SODIUM CHLORIDE (UNII: 451W47IQ8X) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0310-6540-04 4 in 1 CARTON 12/01/2017 1 NDC: 0310-6540-01 0.85 mL in 1 CARTRIDGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 0310-6540-85 1 in 1 CARTON 12/01/2017 2 0.85 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209210 12/01/2017 Labeler - AstraZeneca Pharmaceuticals LP (054743190) Registrant - AstraZeneca PLC (230790719)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.