FOSAMPRENAVIR CALCIUM tablet, film coated
Fosamprenavir Calcium by
Drug Labeling and Warnings
Fosamprenavir Calcium by is a Prescription medication manufactured, distributed, or labeled by Mylan Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FOSAMPRENAVIR CALCIUM TABLETS safely and effectively. See full prescribing information for FOSAMPRENAVIR CALCIUM TABLETS.
FOSAMPRENAVIR CALCIUM tablets, for oral use
Initial U.S. Approval: 2003INDICATIONS AND USAGE
Fosamprenavir calcium tablets are an HIV protease inhibitor indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection. (1)
DOSAGE AND ADMINISTRATION
- Therapy-Naive Adults: Fosamprenavir 1,400 mg twice daily; fosamprenavir 1,400 mg once daily plus ritonavir 200 mg once daily; fosamprenavir 1,400 mg once daily plus ritonavir 100 mg once daily; fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily. (2.2)
- Protease Inhibitor-Experienced Adults: Fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily. (2.2)
- Pregnant Patients: Fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily should only be considered in women who are already on a stable twice-daily regimen of fosamprenavir/ritonavir 700 mg/100 mg prior to pregnancy and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL). (2.2)
- Pediatric Patients (aged at least 4 weeks to 18 years): Dosage should be calculated based on body weight (kg) and should not exceed adult dose. (2.3)
- Hepatic Impairment: Recommended adjustments for patients with mild, moderate, or severe hepatic impairment. (2.4)
Dosing Considerations
- Fosamprenavir calcium tablets may be taken with or without food. (2.1)
DOSAGE FORMS AND STRENGTHS
- 700-mg tablets (3)
CONTRAINDICATIONS
- Hypersensitivity to fosamprenavir or amprenavir (e.g., Stevens-Johnson syndrome). (4)
- Drugs highly dependent on cytochrome P450 (CYP)3A4 for clearance and for which elevated plasma levels may result in serious and/or life-threatening events. (4)
- Review ritonavir contraindications when used in combination. (4)
WARNINGS AND PRECAUTIONS
- The concomitant use of fosamprenavir with ritonavir and certain other drugs may result in known or potentially significant drug interactions. Consult the full prescribing information prior to and during treatment for potential drug interactions. (5.1, 7.3)
- Fosamprenavir should be discontinued for severe skin reactions, including Stevens-Johnson syndrome. (5.2)
- Fosamprenavir should be used with caution in patients with a known sulfonamide allergy. (5.3)
- Use of higher-than-approved doses may lead to transaminase elevations. Patients with hepatitis B or C are at increased risk of transaminase elevations. (5.4)
- Patients receiving fosamprenavir may develop new onset or exacerbations of diabetes mellitus, hyperglycemia (5.5), immune reconstitution syndrome (5.6), increase of body fat (5.7), and elevated triglyceride and cholesterol concentrations (5.8). Monitor cholesterol and triglycerides prior to therapy and periodically thereafter.
- Acute hemolytic anemia has been reported with amprenavir. (5.9)
- Hemophilia: Spontaneous bleeding may occur, and additional factor VIII may be required. (5.10)
- Nephrolithiasis: Cases of nephrolithiasis have been reported with fosamprenavir. (5.11)
ADVERSE REACTIONS
- In adults the most common adverse reactions (incidence greater than or equal to 4%) are diarrhea, rash, nausea, vomiting, and headache. (6.1)
- Vomiting and neutropenia were more frequent in pediatrics than in adults. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Coadministration of fosamprenavir with drugs that induce CYP3A4 may decrease amprenavir (active metabolite) concentrations leading to potential loss of virologic activity. (7, 12.3)
- Coadministration with drugs that inhibit CYP3A4 may increase amprenavir concentrations. (7, 12.3)
- Coadministration of fosamprenavir or fosamprenavir and ritonavir may result in clinically significant interactions with drugs metabolized by CYP3A4. (7)
- Coadministration of fosamprenavir and ritonavir may result in clinically significant interactions with drugs metabolized by CYP2D6. (7)
USE IN SPECIFIC POPULATIONS
Lactation: Breastfeeding is not recommended due to potential for HIV transmission. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Adults
2.3 Pediatric Patients (Aged at Least 4 Weeks to 18 Years)
2.4 Patients with Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Adverse Reactions Due to Drug Interactions
5.2 Skin Reactions
5.3 Sulfa Allergy
5.4 Hepatic Toxicity
5.5 Diabetes/Hyperglycemia
5.6 Immune Reconstitution Syndrome
5.7 Increase in Body Fat
5.8 Lipid Elevations
5.9 Hemolytic Anemia
5.10 Patients with Hemophilia
5.11 Nephrolithiasis
5.12 Resistance/Cross-Resistance
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Cytochrome P450 Inhibitors and Inducers
7.2 Established and Other Potentially Significant Drug Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Therapy-Naive Adult Trials
14.2 Protease Inhibitor-Experienced Adult Trials
14.3 Pediatric Trials
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Fosamprenavir calcium tablets are indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection.
The following points should be considered when initiating therapy with fosamprenavir plus ritonavir in protease inhibitor-experienced patients:
- The protease inhibitor-experienced patient trial was not large enough to reach a definitive conclusion that fosamprenavir plus ritonavir and lopinavir plus ritonavir are clinically equivalent [see Clinical Studies (14.2)].
- Once-daily administration of fosamprenavir plus ritonavir is not recommended for adult protease inhibitor-experienced patients or any pediatric patients [see Dosage and Administration (2.2, 2.3), Clinical Studies (14.2, 14.3)].
- Dosing of fosamprenavir plus ritonavir is not recommended for protease inhibitor-experienced pediatric patients younger than 6 months [see Clinical Pharmacology (12.3)].
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
Fosamprenavir calcium tablets may be taken with or without food.
Higher-than-approved dose combinations of fosamprenavir plus ritonavir are not recommended due to an increased risk of transaminase elevations [see Overdosage (10)].
When fosamprenavir is used in combination with ritonavir, prescribers should consult the full prescribing information for ritonavir.
2.2 Adults
Therapy-Naive Adults
- Fosamprenavir 1,400 mg twice daily (without ritonavir).
- Fosamprenavir 1,400 mg once daily plus ritonavir 200 mg once daily.
-
Fosamprenavir 1,400 mg once daily plus ritonavir 100 mg once daily.
- o Dosing of fosamprenavir 1,400 mg once daily plus ritonavir 100 mg once daily is supported by pharmacokinetic data [see Clinical Pharmacology (12.3)].
-
Fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily.
- o Dosing of fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily is supported by pharmacokinetic and safety data [see Clinical Pharmacology (12.3)].
Protease Inhibitor-Experienced Adults
- Fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily.
Pregnancy
-
Fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily.
- o Dosing of fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily should only be considered in pregnant patients who are already on a stable twice-daily regimen of fosamprenavir/ritonavir 700 mg/100 mg prior to pregnancy and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL). Lower exposures of amprenavir were observed during pregnancy; therefore, viral load should be monitored closely to ensure viral suppression is maintained [see Use in Specific Populations (8.1), Clinical Pharmacology (12.3)]. Data regarding use of other regimens of fosamprenavir (with or without ritonavir) in pregnancy are not available.
2.3 Pediatric Patients (Aged at Least 4 Weeks to 18 Years)
The recommended dosage of fosamprenavir in patients aged at least 4 weeks to 18 years should be calculated based on body weight (kg) and should not exceed the recommended adult dose (Table 1).
Table 1. Twice-Daily Dosage Regimens by Weight for Protease Inhibitor-Naive Pediatric Patients (Aged 4 Weeks and Older) and for Protease Inhibitor-Experienced Pediatric Patients (Aged 6 Months and Older) Using Fosamprenavir Calcium Oral Suspension with Concurrent Ritonavir - * When dosing with ritonavir, do not exceed the adult dose of fosamprenavir 700 mg/ritonavir 100 mg twice-daily dose.
Weight
Twice-Daily Dosage Regimen
< 11 kg
Fosamprenavir 45 mg/kg plus ritonavir 7 mg/kg*
11 kg - < 15 kg
Fosamprenavir 30 mg/kg plus ritonavir 3 mg/kg*
15 kg - < 20 kg
Fosamprenavir 23 mg/kg plus ritonavir 3 mg/kg*
≥ 20 kg
Fosamprenavir 18 mg/kg plus ritonavir 3 mg/kg*
Alternatively, protease inhibitor-naive children aged 2 years and older can be administered fosamprenavir (without ritonavir) 30 mg per kg twice daily.
Fosamprenavir should only be administered to infants born at 38 weeks’ gestation or greater and who have attained a postnatal age of 28 days.
For pediatric patients, pharmacokinetic and clinical data:
- do not support once-daily dosing of fosamprenavir alone or in combination with ritonavir [see Clinical Studies (14.3)].
- do not support administration of fosamprenavir alone or in combination with ritonavir for protease inhibitor-experienced children younger than 6 months [see Clinical Pharmacology (12.3)].
- do not support twice-daily dosing of fosamprenavir without ritonavir in pediatric patients younger than 2 years [see Clinical Pharmacology (12.3)].
Other Dosing Considerations
- When administered without ritonavir, the adult regimen of fosamprenavir calcium tablets 1,400 mg twice daily may be used for pediatric patients weighing at least 47 kg.
- When administered in combination with ritonavir, fosamprenavir calcium tablets may be used for pediatric patients weighing at least 39 kg; ritonavir capsules may be used for pediatric patients weighing at least 33 kg.
2.4 Patients with Hepatic Impairment
See Clinical Pharmacology (12.3).
Mild Hepatic Impairment (Child-Pugh Score Ranging from 5 to 6)
Fosamprenavir should be used with caution at a reduced dosage of 700 mg twice daily without ritonavir (therapy-naive) or 700 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced).
Moderate Hepatic Impairment (Child-Pugh Score Ranging from 7 to 9)
Fosamprenavir should be used with caution at a reduced dosage of 700 mg twice daily without ritonavir (therapy-naive), or 450 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced).
Severe Hepatic Impairment (Child-Pugh Score Ranging from 10 to 15)
Fosamprenavir should be used with caution at a reduced dosage of 350 mg twice daily without ritonavir (therapy-naive) or 300 mg twice daily plus ritonavir 100 mg once daily (therapy-naive or protease inhibitor-experienced).
There are no data to support dosing recommendations for pediatric patients with hepatic impairment.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Fosamprenavir calcium tablets are contraindicated in patients with previously demonstrated clinically significant hypersensitivity (e.g., Stevens-Johnson syndrome) to any of the components of this product or to amprenavir.
- Fosamprenavir calcium tablets are contraindicated when coadministered with drugs that are highly dependent on cytochrome P450 (CYP)3A4 for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events. These drugs and other contraindicated drugs (which may lead to reduced efficacy of fosamprenavir and possible resistance) are listed below [see Drug Interactions (7), Clinical Pharmacology (12.3)]. The list of contraindicated drugs applies to the use of fosamprenavir with or without ritonavir, unless otherwise indicated. If fosamprenavir is coadministered with ritonavir, reference should be made to the full prescribing information for ritonavir for additional contraindications.
-
Fosamprenivir is contraindicated when coadministered with the following drugs:
- o Alpha 1-adrenoreceptor antagonist: Alfuzosin
- o Antiarrhythmics: Flecainide (with ritonavir), propafenone (with ritonavir)
- o Antimycobacterial: Rifampin
- o Antipsychotic: Lurasidone (with ritonavir), pimozide
- o Ergot derivatives: Dihydroergotamine, ergonovine, ergotamine, methylergonovine
- o GI motility agent: Cisapride
- o Herbal product: St. John’s wort (Hypericum perforatum)
- o Lipid modifying agents: Lomitapide, lovastatin, simvastatin
- o Non-nucleoside reverse transcriptase inhibitor: Delavirdine
- o PDE5 inhibitor: Sildenafil (REVATIO®) (for treatment of pulmonary arterial hypertension)
- o Sedative/hypnotics: Midazolam, triazolam
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Adverse Reactions Due to Drug Interactions
Initiation of fosamprenavir/ritonavir, a CYP3A inhibitor, in patients receiving medications metabolized by CYP3A, or initiation of medications metabolized by CYP3A in patients already receiving fosamprenavir/ritonavir may increase plasma concentrations of medications metabolized by CYP3A. Initiation of medications that inhibit or induce CYP3A may increase or decrease concentrations of fosamprenavir/ritonavir, respectively. These interactions may lead to:
- clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal events from greater exposures of concomitant medications.
- clinically significant adverse reactions from greater exposures of fosamprenavir/ritonavir.
- loss of therapeutic effect of fosamprenavir/ritonavir and possible development of resistance.
See Table 6 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations [see Drug Interactions (7)]. Consider the potential for drug interactions prior to and during therapy with fosamprenavir/ritonavir; review concomitant medications during therapy with fosamprenavir/ritonavir; and monitor for the adverse reactions associated with the concomitant medications [see Contraindications (4), Drug Interactions (7)].
5.2 Skin Reactions
Severe and life-threatening skin reactions, including 1 case of Stevens-Johnson syndrome among 700 subjects treated with fosamprenavir in clinical trials. Treatment with fosamprenavir should be discontinued for severe or life-threatening rashes and for moderate rashes accompanied by systemic symptoms [see Adverse Reactions (6)].
5.3 Sulfa Allergy
Fosamprenavir should be used with caution in patients with a known sulfonamide allergy. Fosamprenavir contains a sulfonamide moiety. The potential for cross-sensitivity between drugs in the sulfonamide class and fosamprenavir is unknown. In a clinical trial of fosamprenavir used as the sole protease inhibitor, rash occurred in 2 of 10 subjects (20%) with a history of sulfonamide allergy compared with 42 of 126 subjects (33%) with no history of sulfonamide allergy. In 2 clinical trials of fosamprenavir plus low-dose ritonavir, rash occurred in 8 of 50 subjects (16%) with a history of sulfonamide allergy compared with 50 of 412 subjects (12%) with no history of sulfonamide allergy.
5.4 Hepatic Toxicity
Use of fosamprenavir with ritonavir at higher-than-recommended dosages may result in transaminase elevations and should not be used [see Dosage and Administration (2), Overdosage (10)]. Patients with underlying hepatitis B or C or marked elevations in transaminases prior to treatment may be at increased risk for developing or worsening of transaminase elevations. Appropriate laboratory testing should be conducted prior to initiating therapy with fosamprenavir and patients should be monitored closely during treatment.
5.5 Diabetes/Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV-1– infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and causal relationships between protease inhibitor therapy and these events have not been established.
5.6 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including fosamprenavir. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.7 Increase in Body Fat
Increase of body fat has been observed in patients receiving protease inhibitors, including fosamprenavir. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.8 Lipid Elevations
Treatment with fosamprenavir plus ritonavir has resulted in increases in the concentration of triglycerides and cholesterol [see Adverse Reactions (6)]. Triglyceride and cholesterol testing should be performed prior to initiating therapy with fosamprenavir and at periodic intervals during therapy. Lipid disorders should be managed as clinically appropriate [see Drug Interactions (7)].
5.10 Patients with Hemophilia
There have been reports of spontaneous bleeding in patients with hemophilia A and B treated with protease inhibitors. In some patients, additional factor VIII was required. In many of the reported cases, treatment with protease inhibitors was continued or restarted. A causal relationship between protease inhibitor therapy and these episodes has not been established.
5.11 Nephrolithiasis
Cases of nephrolithiasis were reported during postmarketing surveillance in HIV-1– infected patients receiving fosamprenavir. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made. If signs or symptoms of nephrolithiasis occur, temporary interruption or discontinuation of therapy may be considered.
5.12 Resistance/Cross-Resistance
Because the potential for HIV cross-resistance among protease inhibitors has not been fully explored, it is unknown what effect therapy with fosamprenavir will have on the activity of subsequently administered protease inhibitors. Fosamprenavir has been studied in patients who have experienced treatment failure with protease inhibitors [see Clinical Studies (14.2)].
-
6 ADVERSE REACTIONS
- Severe or life-threatening skin reactions have been reported with the use of fosamprenavir [see Warnings and Precautions (5.2)].
- The most common moderate to severe adverse reactions in clinical trials of fosamprenavir were diarrhea, rash, nausea, vomiting, and headache.
- Treatment discontinuation due to adverse events occurred in 6.4% of subjects receiving fosamprenavir and in 5.9% of subjects receiving comparator treatments. The most common adverse reactions leading to discontinuation of fosamprenavir (incidence less than or equal to 1% of subjects) included diarrhea, nausea, vomiting, AST increased, ALT increased, and rash.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adult Trials
The data for the 3 active-controlled clinical trials described below reflect exposure of 700 HIV-1– infected subjects to fosamprenavir calcium tablets, including 599 subjects exposed to fosamprenavir for greater than 24 weeks, and 409 subjects exposed for greater than 48 weeks. The population age ranged from 17 to 72 years. Of these subjects, 26% were female, 51% white, 31% black, 16% American Hispanic, and 70% were antiretroviral-naive. Sixty-one percent received fosamprenavir 1,400 mg once daily plus ritonavir 200 mg once daily; 24% received fosamprenavir 1,400 mg twice daily; and 15% received fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily.
Selected adverse reactions reported during the clinical efficacy trials of fosamprenavir are shown in Tables 2 and 3. Each table presents adverse reactions of moderate or severe intensity in subjects treated with combination therapy for up to 48 weeks.
Table 2. Selected Moderate/Severe Clinical Adverse Reactions Reported in Greater than or Equal to 2% of Antiretroviral-Naive Adult Subjects - * All subjects also received abacavir and lamivudine twice daily.
Adverse Reaction
APV30001*
APV30002*
Fosamprenavir
1,400 mg
Twice Daily(n = 166)
Nelfinavir
1,250 mg
Twice Daily(n = 83)
Fosamprenavir
1,400 mg and Ritonavir
200 mg
Once Daily(n = 322)
Nelfinavir
1,250 mg
Twice Daily(n = 327)
Gastrointestinal
Diarrhea
5%
18%
10%
18%
Nausea
7%
4%
7%
5%
Vomiting
2%
4%
6%
4%
Abdominal pain
1%
0%
2%
2%
Skin
Rash
8%
2%
3%
2%
General disorders
Fatigue
2%
1%
4%
2%
Nervous system
Headache
2%
4%
3%
3%
Table 3. Selected Moderate/Severe Clinical Adverse Reactions Reported in Greater than or Equal to 2% of Protease Inhibitor-Experienced Adult Subjects (Trial APV30003) - * All subjects also received 2 reverse transcriptase inhibitors.
Adverse Reaction
Fosamprenavir
700 mg and
Ritonavir 100 mg Twice Daily*
(n = 106)
Lopinavir 400 mg and
Ritonavir 100 mg Twice Daily*
(n = 103)
Gastrointestinal
Diarrhea
13%
11%
Nausea
3%
9%
Vomiting
3%
5%
Abdominal pain
< 1%
2%
Skin
Rash
3%
0%
Nervous system
Headache
4%
2%
Skin rash (without regard to causality) occurred in approximately 19% of subjects treated with fosamprenavir in the pivotal efficacy trials. Rashes were usually maculopapular and of mild or moderate intensity, some with pruritus. Rash had a median onset of 11 days after initiation of fosamprenavir and had a median duration of 13 days. Skin rash led to discontinuation of fosamprenavir in less than 1% of subjects. In some subjects with mild or moderate rash, dosing with fosamprenavir was often continued without interruption; if interrupted, reintroduction of fosamprenavir generally did not result in rash recurrence.
The percentages of subjects with Grade 3 or 4 laboratory abnormalities in the clinical efficacy trials of fosamprenavir are presented in Tables 4 and 5.
Table 4. Grade 3/4 Laboratory Abnormalities Reported in Greater than or Equal to 2% of Antiretroviral-Naive Adult Subjects in Trials APV30001 and APV30002 ULN = Upper limit of normal. - * All subjects also received abacavir and lamivudine twice daily.
- † Fasting specimens.
Laboratory Abnormality
APV30001*
APV30002*
Fosamprenavir
1,400 mg
Twice Daily(n = 166)
Nelfinavir
1,250 mg
Twice Daily(n = 83)
Fosamprenavir
1,400 mg and
Ritonavir
200 mg
Once Daily(n = 322)
Nelfinavir
1,250 mg
Twice Daily(n = 327)
ALT (> 5 x ULN)
6%
5%
8%
8%
AST (> 5 x ULN)
6%
6%
6%
7%
Serum lipase (> 2 x ULN)
8%
4%
6%
4%
Triglycerides†
(> 750 mg/dL)
0%
1%
6%
2%
Neutrophil count, absolute
(< 750 cells/mm3)
3%
6%
3%
4%
The incidence of Grade 3 or 4 hyperglycemia in antiretroviral-naive subjects who received fosamprenavir in the pivotal trials was less than 1%.
Table 5. Grade 3/4 Laboratory Abnormalities Reported in Greater than or Equal to 2% of Protease Inhibitor-Experienced Adult Subjects in Trial APV30003 ULN = Upper limit of normal. - * All subjects also received 2 reverse transcriptase inhibitors.
- † Fasting specimens.
- ‡ n = 100 for fosamprenavir plus ritonavir, n = 98 for lopinavir plus ritonavir.
Laboratory Abnormality
Fosamprenavir
700 mg and
Ritonavir 100 mg
Twice Daily.*(n = 104)
Lopinavir 400 mg and
Ritonavir 100 mg Twice Daily*
(n = 103)
Triglycerides† (> 750 mg/dL)
11%‡
6%‡
Serum lipase (> 2 x ULN)
5%
12%
ALT (> 5 x ULN)
4%
4%
AST (> 5 x ULN)
4%
2%
Glucose (> 251 mg/dL)
2%‡
2%‡
Pediatric Trials
Fosamprenavir with and without ritonavir was studied in 237 HIV-1– infected pediatric subjects aged at least 4 weeks to 18 years in 3 open-label trials; APV20002, APV20003, and APV29005 [see Clinical Studies (14.3)]. Vomiting and neutropenia occurred more frequently in pediatric subjects compared with adults. Other adverse events occurred with similar frequency in pediatric subjects compared with adults.
The frequency of vomiting among pediatric subjects receiving fosamprenavir twice daily with ritonavir was 20% in subjects aged at least 4 weeks to younger than 2 years and 36% in subjects aged 2 to 18 years compared with 10% in adults. The frequency of vomiting among pediatric subjects receiving fosamprenavir twice daily without ritonavir was 60% in subjects aged 2 to 5 years compared with 16% in adults.
The median duration of drug-related vomiting episodes in APV29005 was 1 day (range: 1 to 3 days), in APV20003 was 16 days (range: 1 to 38 days), and in APV20002 was 9 days (range: 4 to 13 days). Vomiting was treatment limiting in 4 pediatric subjects across all 3 trials.
The incidence of Grade 3 or 4 neutropenia (neutrophils less than 750 cells per mm3) seen in pediatric subjects treated with fosamprenavir with and without ritonavir was higher (15%) than the incidence seen in adult subjects (3%). Grade 3/4 neutropenia occurred in 10% (5 of 51) of subjects aged at least 4 weeks to younger than 2 years and 16% (28 of 170) of subjects aged 2 to 18 years.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of fosamprenavir. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to fosamprenavir.
Cardiac Disorders: Myocardial infarction.
Metabolism and Nutrition Disorders: Hypercholesterolemia.
Nervous System Disorders: Oral paresthesia.
Skin and Subcutaneous Tissue Disorders: Angioedema.
Urogenital: Nephrolithiasis.
-
7 DRUG INTERACTIONS
7.1 Cytochrome P450 Inhibitors and Inducers
Amprenavir, the active metabolite of fosamprenavir, is an inhibitor of CYP3A4 metabolism and therefore should not be administered concurrently with medications with narrow therapeutic windows that are substrates of CYP3A4. Data also suggest that amprenavir induces CYP3A4.
Amprenavir is metabolized by CYP3A4. Coadministration of fosamprenavir and drugs that induce CYP3A4, such as rifampin, may decrease amprenavir concentrations and reduce its therapeutic effect. Coadministration of fosamprenavir and drugs that inhibit CYP3A4 may increase amprenavir concentrations and increase the incidence of adverse effects.
The potential for drug interactions with fosamprenavir changes when fosamprenavir is coadministered with the potent CYP3A4 inhibitor ritonavir. The magnitude of CYP3A4-mediated drug interactions (effect on amprenavir or effect on coadministered drug) may change when fosamprenavir is coadministered with ritonavir. Because ritonavir is a CYP2D6 inhibitor, clinically significant interactions with drugs metabolized by CYP2D6 are possible when coadministered with fosamprenavir plus ritonavir. Ritonavir also appears to induce CYP3A, CYP1A2, CYP2C9, CYP2C19, and CYP2B6, as well as other enzymes, including glucuronosyl transferase.
There are other agents that may result in serious and/or life-threatening drug interactions [see Contraindications (4)].
7.2 Established and Other Potentially Significant Drug Interactions
If fosamprenavir is used in combination with ritonavir, see full prescribing information for ritonavir for additional information on drug interactions [see Contraindications (4), Clinical Pharmacology (12.3)].
Table 6 provides a listing of established or potentially clinically significant drug interactions. Information in the table applies to fosamprenavir with or without ritonavir, unless otherwise indicated.
Table 6. Established and Other Potentially Significant Drug Interactions - * See Clinical Pharmacology (12.3)Tables 10, 11, 12, or 13 for magnitude of interaction.
Concomitant Drug Class: Drug Name
Effect on Concentration of Amprenavir or Concomitant Drug
Clinical Comment
HCV/HIV-Antiviral Agents
HCV protease inhibitor:
Boceprevir
Fosamprenavir: ↓ Amprenavir (predicted)
↔ or ↓ Boceprevir (predicted)
Fosamprenavir/
ritonavir:
↓ Amprenavir (predicted)
↓ Boceprevir (predicted)Coadministration of fosamprenavir or fosamprenavir/ritonavir and boceprevir is not recommended.
HCV protease inhibitor:
Simeprevir
Fosamprenavir:
↔ Amprenavir (predicted)
↑ or ↓ Simeprevir (predicted)
Fosamprenavir/
ritonavir: ↔ Amprenavir (predicted)
↑ Simeprevir (predicted)
Coadministration of fosamprenavir or fosamprenavir/ritonavir and simeprevir is not recommended.
HCV protease inhibitor:
Paritaprevir (coformulated with ritonavir and ombitasvir and coadministered with dasabuvir)
Fosamprenavir:
↑ Amprenavir (predicted)
↑ or ↔ Paritaprevir (predicted)
Fosamprenavir/
ritonavir: ↑ or ↔ Amprenavir (predicted)
↑ Paritaprevir (predicted)
Appropriate doses of the combinations with respect to safety and efficacy have not been established.
Fosamprenavir 1,400 mg once daily may be considered when coadministered with paritaprevir/ritonavir/ombitasvir/dasabuvir.
Coadministration of fosamprenavir/ritonavir and paritaprevir/ritonavir/ombitasvir/ dasabuvir is not recommended.
Non-nucleoside reverse transcriptase inhibitor:
Delavirdine*
Fosamprenavir:
↑ Amprenavir
↓ Delavirdine
Fosamprenavir/
ritonavir:
↑ Amprenavir
↓ Delavirdine
Coadministration is contraindicated as it may lead to loss of virologic response and possible resistance to delavirdine [see Contraindications (4)].
Non-nucleoside reverse transcriptase inhibitor:
Efavirenz*
Fosamprenavir:
↓ Amprenavir
Appropriate doses of the combinations with respect to safety and efficacy have not been established.
Fosamprenavir/
ritonavir:
↓ Amprenavir
An additional 100 mg/day (300 mg total) of ritonavir is recommended when efavirenz is administered with fosamprenavir/ritonavir once daily. No change in the ritonavir dose is required when efavirenz is administered with fosamprenavir plus ritonavir twice daily.
Non-nucleoside reverse transcriptase inhibitor:
Nevirapine*
Fosamprenavir:
↓ Amprenavir
↑ Nevirapine
Coadministration of nevirapine and fosamprenavir without ritonavir is not recommended.
Fosamprenavir/
ritonavir:
↓ Amprenavir
↑ Nevirapine
No dosage adjustment required when nevirapine is administered with fosamprenavir/ritonavir twice daily.
The combination of nevirapine administered with fosamprenavir/ritonavir once-daily regimen has not been studied.HIV protease inhibitor:
Atazanavir*
Fosamprenavir:
Interaction has not been evaluated.
Fosamprenavir/ritonavir:
↓ Atazanavir
↔ Amprenavir
Appropriate doses of the combinations with respect to safety and efficacy have not been established.
HIV protease inhibitors:
Fosamprenavir:
↑ Amprenavir
Effect on indinavir and nelfinavir is not well established.
Fosamprenavir/ritonavir: Interaction has not been evaluated.
Appropriate doses of the combinations with respect to safety and efficacy have not been established.
HIV protease inhibitors:
Lopinavir/ritonavir*
↓ Amprenavir
↓ Lopinavir
An increased rate of adverse events has been observed. Appropriate doses of the combinations with respect to safety and efficacy have not been established.
HIV protease inhibitor:
Saquinavir*
Fosamprenavir:
↓ Amprenavir
Effect on saquinavir is not well established.
Fosamprenavir/ritonavir: Interaction has not been evaluated.
Appropriate doses of the combination with respect to safety and efficacy have not been established.
HIV integrase inhibitor:
Raltegravir*
Fosamprenavir:
↓ Amprenavir↓ Raltegravir
Fosamprenavir/ritonavir:
↓ Amprenavir
↓ Raltegravir
Appropriate doses of the combination with respect to safety and efficacy have not been established.
HIV integrase inhibitor:
Dolutegravir*Fosamprenavir/
ritonavir:
↓ DolutegravirThe recommended dose of dolutegravir is 50 mg twice daily when coadministered with fosamprenavir/ritonavir.
Use an alternative combination where possible in patients with known or suspected integrase inhibitor resistance.
HIV CCR5 co-receptor antagonist:
Maraviroc*
Fosamprenavir/
ritonavir:
↓ Amprenavir
↑ Maraviroc
No dosage adjustment required for fosamprenavir/ritonavir. The recommended dose of maraviroc is 150 mg twice daily when coadministered with fosamprenavir/ritonavir. Fosamprenavir should be given with ritonavir when coadministered with maraviroc.
Other Agents
Alpha 1-adrenoreceptor
antagonist:
Alfuzosin
↑ Alfuzosin
Coadministration is contraindicated due
to potential hypotension [see
Antacid:
MAALOX TC®
↓ Amprenavir
Use with caution when administered at
the same time. Fosamprenavir may be less
effective due to decreased amprenavir
plasma concentrations.
Staggered coadministration of antacids
and fosamprenavir has not been evaluated.
Antiarrhythmics:
Amiodarone, disopyramide, lidocaine (systemic), and quinidine
↑ Antiarrhythmics
Therapeutic concentration monitoring, if available, is recommended for antiarrhythmics.
Antiarrhythmics:
Flecainide, propafenone
↑ Antiarrhythmics
Coadministration is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias if fosamprenavir is co-prescribed with ritonavir [see Contraindications (4)].
Anticoagulant:
Warfarin
Concentrations of warfarin may be affected. It is recommended that INR (international normalized ratio) be monitored.
Anticonvulsants: Carbamazepine, phenobarbital, phenytoin
Fosamprenavir:
↓ Amprenavir
Use with caution. Fosamprenavir may be less effective due to decreased amprenavir plasma concentrations in patients taking these agents concomitantly.
Phenytoin*
Fosamprenavir/
ritonavir:
↑ Amprenavir
↓ Phenytoin
Plasma phenytoin concentrations should be monitored and phenytoin dose should be increased as appropriate. No change in fosamprenavir/ritonavir dose is recommended.
Antidepressant:
Paroxetine, trazodone
↓ Paroxetine
Any paroxetine dose adjustment should be guided by clinical effect (tolerability and efficacy).
↑ Trazodone
Adverse events of nausea, dizziness, hypotension, and syncope have been observed following coadministration of trazodone and ritonavir. If trazodone is used with a CYP3A4 inhibitor such as fosamprenavir, the combination should be used with caution and a lower dose of trazodone should be considered.
Antifungals:
Ketoconazole*,
itraconazole
↑ Ketoconazole
↑ Itraconazole
Increase monitoring for adverse events.
Fosamprenavir: Dose reduction of ketoconazole or itraconazole may be needed for patients receiving more than 400 mg ketoconazole or itraconazole per day.
Fosamprenavir/ritonavir: High doses of ketoconazole or itraconazole (greater than 200 mg/day) are not recommended.Anti-gout:
Colchicine
↑ Colchicine
Patients with renal or hepatic impairment should not be given colchicine with fosamprenavir/ritonavir.
Fosamprenavir/ritonavir and coadministration of colchicine:
Treatment of gout flares: 0.6 mg (1 tablet) x 1 dose, followed by 0.3 mg (half tablet) 1 hour later. Dose to be repeated no earlier than 3 days.
Prophylaxis of gout flares: If the original regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg once a day. If the original regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once every other day.
Treatment of familial Mediterranean fever (FMF): Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day).
Fosamprenavir and coadministration of colchicine:
Treatment of gout flares: 1.2 mg (2 tablets) x 1 dose. Dose to be repeated no earlier than 3 days.
Prophylaxis of gout flares: If the original regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg twice a day or 0.6 mg once a day.
If the original regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once a day.
Treatment of FMF: Maximum daily dose of 1.2 mg (may be given as 0.6 mg twice a day).Antimycobacterial:
Rifabutin*
↑ Rifabutin and rifabutin metabolite
A complete blood count should be performed weekly and as clinically indicated to monitor for neutropenia.
Fosamprenavir: A dosage reduction of rifabutin by at least half the recommended dose is required.
Fosamprenavir/ritonavir: Dosage reduction of rifabutin by at least 75% of the usual dose of 300 mg/day is recommended (a maximum dose of 150 mg every other day or 3 times per week).Antimycobacterial:
Rifampina
↓ Amprenavir
Coadministration is contraindicated as it may lead to loss of virologic response and possible resistance to fosamprenavir or to the class of protease inhibitors [see Contraindications (4)].
Antineoplastics:
Dasatinib, nilotinib,
ibrutinib, vinblastine,
everolimus
↑ Antineoplastics
Fosamprenavir or fosamprenavir/ritonavir may increase plasma concentrations of antineoplastics metabolized by CYP3A, potentially increasing the risk of adverse events typically associated with these medications. In case of coadministration, please refer to relevant prescribing information for these medications.
Antipsychotics:
Lurasidone
↑ Lurasidone
Fosamprenavir: If coadministration is necessary, reduce the lurasidone dose. Refer to the lurasidone prescribing information for concomitant use with moderate CYP3A4 inhibitors.
Fosamprenavir/ritonavir: Use of lurasidone is contraindicated due to potential for serious and/or life-threatening reactions [see Contraindications (4)].
Pimozide
↑ Pimozide
Coadministration is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)].
Quetiapine
↑ Quetiapine
Fosamprenavir/ritonavir:
Initiation of fosamprenavir with ritonavir in patients taking quetiapine: Consider alternative antiretroviral therapy to avoid increases in quetiapine drug exposures. If coadministration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring.
Initiation of quetiapine in patients taking fosamprenavir with ritonavir: Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine.
Benzodiazepines:
Alprazolam, clorazepate, diazepam, flurazepam
↑ Benzodiazepines
Clinical significance is unknown. A decrease in benzodiazepine dose may be needed.
Calcium channel blockers:
Diltiazem, felodipine, nifedipine, nicardipine, nimodipine, verapamil, amlodipine, nisoldipine, isradipine
↑ Calcium channel blockers
Use with caution. Clinical monitoring of patients is recommended.
Corticosteroid:
Dexamethasone
↓ Amprenavir
Use with caution. Fosamprenavir may be less effective due to decreased amprenavir plasma concentrations.
Endothelin-receptor antagonist:
Bosentan
↑ Bosentan
Coadministration of bosentan in patients on fosamprenavir:In patients who have been receiving fosamprenavir for at least 10 days, start bosentan at 62.5 mg once daily or every other day based upon individual tolerability.
Coadministration of fosamprenavir in patients on bosentan:Discontinue use of bosentan at least 36 hours prior to initiation of fosamprenavir.
After at least 10 days following the initiation of fosamprenavir, resume bosentan at 62.5 mg once daily or every other day based upon individual tolerability.Ergot derivatives:
Dihydroergotamine, ergonovine, ergotamine,
methylergonovine
↑ Ergot derivatives
Coadministration is contraindicated due to potential for serious and/or life-threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues [see Contraindications (4)].
GI motility agent:
Cisapride
↑ Cisapride
Coadministration is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias [see Contraindications (4)].
Herbal product:
St. John’s wort
(Hypericum perforatum)
↓ Amprenavir
Coadministration is contraindicated as it may lead to loss of virologic response and possible resistance to fosamprenavir or to the class of protease inhibitors [see Contraindications (4)].
Histamine H2-receptor
antagonists:
Cimetidine, famotidine, nizatidine, ranitidine*
Fosamprenavir:
↓ Amprenavir
Fosamprenavir/ritonavir: Interaction not evaluated
Use with caution. Fosamprenavir may be less effective due to decreased amprenavir plasma concentrations.
Immunosuppressants:
Cyclosporine, tacrolimus, sirolimus
↑ Immunosuppressants
Therapeutic concentration monitoring is recommended for immunosuppressant agents.
Inhaled beta-agonist:
Salmeterol
↑ Salmeterol
Concurrent administration of salmeterol with fosamprenavir is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations, and sinus tachycardia.
Inhaled/nasal steroid:
Fluticasone
Fosamprenavir:
↑ Fluticasone
Fosamprenavir/
ritonavir:
↑ Fluticasone
Use with caution. Consider alternatives to fluticasone, particularly for long-term use.
May result in significantly reduced serum cortisol concentrations. Systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression have been reported during postmarketing use in patients receiving ritonavir and inhaled or intranasally administered fluticasone. Coadministration of fluticasone and fosamprenavir/ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects.Lipid Modifying Agents:
HMG-CoA reductase inhibitors:
Atorvastatina
↑ Atorvastatin
Titrate atorvastatin dose carefully and use the lowest necessary dose; do not exceed atorvastatin 20 mg/day.
Lovastatin, simvastatin
↑ Lovastatin
↑ Simvastatin
Coadministration with lovastatin or
simvastatin is contraindicated due to
potential for serious reactions such as risk
of myopathy including rhabdomyolysis
[see Contraindications (4)].
Other lipid modifying agents:
Lomitapide
↑ Lomitapide
Coadministration with lomitapide is
contraindicated due to potential for
markedly increased transaminases.
Narcotic analgesic:
Methadone
↓ Methadone
Data suggest that the interaction is not clinically relevant; however, patients should be monitored for opiate withdrawal symptoms.
Fentanyl
↑ Fentanyl
Careful monitoring of therapeutic effects
and adverse effects of fentanyl (including
potentially fatal respiratory depression) is
recommended.
Oral contraceptives:
Ethinyl estradiol/norethindrone*
Fosamprenavir:
↓ Amprenavir
↓ Ethinyl estradiol
Alternative methods of non-hormonal contraception are recommended.
May lead to loss of virologic response.*
Fosamprenavir/
ritonavir:
↓ Ethinyl estradiol
Increased risk of transaminase elevations. No data are available on the use of fosamprenavir/ritonavir with other hormonal therapies, such as hormone replacement therapy (HRT) for postmenopausal women.
PDE5 inhibitors:
Sildenafil, tadalafil, vardenafil
↑ Sildenafil
↑ Tadalafil
↑ Vardenafil
May result in an increase in PDE5 inhibitor-associated adverse events, including hypotension, syncope, visual disturbances, and priapism.
Use of PDE5 inhibitors for pulmonary arterial hypertension (PAH): Use of sildenafil (REVATIO) is contraindicated when used for the treatment of PAH. A safe and effective dose has not been established when used with fosamprenavir. There is increased potential for sildenafil-associated adverse events (which include visual disturbances, hypotension, prolonged erection, and syncope) [see Contraindications (4)].
-
The following dose adjustments are recommended for use of tadalafil (ADCIRCA®) with fosamprenavir:
Coadministration of ADCIRCA in patients on fosamprenavir: In patients receiving fosamprenavir for at least one week, start ADCIRCA at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability.
Coadministration of fosamprenavir in patients on ADCIRCA: Avoid use of ADCIRCA during the initiation of fosamprenavir. Stop ADCIRCA at least 24 hours prior to starting fosamprenavir. After at least one week following the initiation of fosamprenavir, resume ADCIRCA at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability.
Use of PDE5 inhibitors for erectile dysfunction:
Fosamprenavir:
Sildenafil: 25 mg every 48 hours.
Tadalafil: no more than 10 mg every 72 hours.
Vardenafil: no more than 2.5 mg every 24 hours.
Fosamprenavir/ritonavir:
Sildenafil: 25 mg every 48 hours.
Tadalafil: no more than 10 mg every 72 hours.
Vardenafil: no more than 2.5 mg every 72 hours.
Use with increased monitoring for adverse events.
Proton pump inhibitors:
Esomeprazole* , lansoprazole, omeprazole, pantoprazole, rabeprazole
Fosamprenavir:
↔ Amprenavir
↑ Esomeprazole
Fosamprenavir/
ritonavir:
↔ Amprenavir
↔ Esomeprazole
Proton pump inhibitors can be administered at the same time as a dose of fosamprenavir with no change in plasma amprenavir concentrations.
Sedative/hypnotics:
Midazolam, triazolam
↑ Midazolam
↑ Triazolam
Coadministration is contraindicated due to potential for serious and/or life-threatening reactions such as prolonged or increased sedation or respiratory depression [see Contraindications (4)].
Tricyclic antidepressants:
Amitriptyline, imipramine
↑ Tricyclics
Therapeutic concentration monitoring is recommended for tricyclic antidepressants.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to fosamprenavir during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Limited data are available for use of fosamprenavir in pregnancy. Fosamprenavir 700 mg twice daily taken with ritonavir 100 mg twice daily should only be considered in pregnant patients who are already on a stable twice-daily regimen of fosamprenavir/ritonavir 700 mg/100 mg prior to pregnancy, and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) (see Clinical Considerations and Data).
There are insufficient human data on the use of fosamprenavir during pregnancy to adequately assess a drug-associated risk for birth defects and miscarriage. Given the limited number of pregnancies exposed to fosamprenavir-based regimens, no conclusions can be drawn on the safety of fosamprenavir in pregnancy. The background risk for major birth defects and miscarriage for the indicated population is unknown. The background rate for major birth defects in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) is 2.7% (see Data). The estimated background rate of miscarriage in clinically recognized pregnancies in the U.S. general population is 15% to 20%.
In animal reproduction studies, no evidence of major adverse developmental outcomes was observed following oral administration of fosamprenavir. Systemic exposure to amprenavir (the active ingredient) was less than (rabbits) or up to 2 times (rats) those in humans at the maximum recommended human dose (MRHD) with or without ritonavir. In contrast, oral administration of amprenavir was associated with abortions in pregnant rabbits at doses that produced approximately one-twentieth the human exposure at the MRHD.
In the rat pre- and postnatal development study, toxicities to the offspring, including reduced survival and reproductive performance, were observed at maternal systemic exposures (AUC) to amprenavir that were approximately 2 times the exposure in humans at the MRHD of fosamprenavir alone or approximately the same as those seen in humans following administration of the MRHD of fosamprenavir in combination with ritonavir (see Data).
Clinical Considerations
Virologic Monitoring During Pregnancy and the Postpartum Period
Based on limited data on the use of fosamprenavir during pregnancy, no dosage adjustments are required for pregnant patients who are already on a stable twice-daily regimen of fosamprenavir 700 mg taken with ritonavir 100 mg prior to pregnancy, and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)]. In a clinical trial of 10 HIV-1–infected pregnant women treated with fosamprenvir 700 mg taken with ritonavir 100 mg twice daily through postpartum, total amprenavir exposures were lower during pregnancy compared with the postpartum period. Therefore, viral load should be monitored closely to ensure viral suppression is maintained [see Data, Dosage and Administration (2.2), Clinical Pharmacology (12.3)]. Pregnancy data with other dosage regimens of fosamprenavir (with or without ritonavir) are not available.
Data
Human Data
Fosamprenavir 700 mg taken with ritonavir 100 mg twice daily in combination with a background regimen was evaluated in a clinical trial of 10 HIV-1–infected pregnant women during the second and third trimesters and postpartum. Subjects initiated fosamprenavir/ritonavir during pregnancy at a median of 19 weeks’ gestation; 4 had undetectable HIV-1 RNA viral load (less than 50 copies/mL) at the time of initiation. Amprenavir pharmacokinetics and placental transfer were studied during the second trimester (n = 6) or third trimester (n = 9) and postpartum (n = 9). Pregnancy outcomes were available for all 10 subjects, among which 1 twin pregnancy was included. Compared to the postpartum period, geometric mean amprenavir AUC was 35% lower in the second trimester and 25% lower in the third trimester. The amprenavir geometric mean ratio (95% CI) of fetal cord to maternal peripheral plasma concentration (n = 7) was 0.27 (0.24 to 0.30) [see Clinical Pharmacology (12.3)]. At delivery, 9 subjects had HIV-1 viral load less than 50 copies/mL, and 1 subject had a viral load of 111 copies/mL. All 11 infants born had test results that were negative for HIV-1 at the time of delivery and through 12 months postpartum. There were no new safety findings compared with the known safety profile of fosamprenavir/ritonavir in HIV-1–infected adults.
Based on prospective reports to the APR of approximately 146 live births following exposure to fosamprenavir-containing regimens, there were 2 birth defects reported in 109 first trimester exposures and 2 birth defects reported in 36 second and third trimester exposures. The background rate for major birth defects is 2.7% in a U.S. reference population of the MACDP. Prospective reports from the APR of overall major birth defects in pregnancies exposed to fosamprenavir are compared with the U.S. background major birth defect rate. Methodological limitations of the APR include the use of MACDP as the external comparator group. Limitations of using an external comparator include differences in methodology and populations as well as confounding due to the underlying disease.
Animal Data
Fosamprenavir was administered orally to pregnant rats (300, 820, or 2,240 mg per kg per day) and rabbits (74.8, 224.3, or 672.8 mg per kg per day) on Gestation Days 6 to 17 and Days 7 to 20, respectively. No major adverse effects on embryo-fetal development were observed at these dose levels, resulting in exposures (AUC0-24 h) approximately 2 times (rats) and 0.8 times (rabbits) human exposures at the MRHD of fosamprenavir alone or 0.7 times (rats) and 0.3 times (rabbits) human exposures at the MRHD of fosamprenavir in combination with ritonavir. However, increased incidence of abortion was observed in rabbits administered a maternally toxic dose of fosamprenavir (672.8 mg per kg per day). In a study where amprenavir was administered orally to pregnant rabbits (25, 50, or 100 mg per kg per day) on Gestation Days 8 to 20, increased abortions and an increased incidence of minor skeletal variations (deficient ossification of the femur, humerus, and trochlea) were observed at doses that produced approximately one-twentieth the exposure seen at the MRHD.
In the rat pre- and postnatal development study, fosamprenavir was administered orally (300, 820, or 2,240 mg per kg per day) on Gestation Day 6 to Lactation/Postpartum Day 20. Fosamprenavir caused a reduction in pup survival and body weights. In surviving female offspring from the high-dose group, an increased time to successful mating, an increased length of gestation, a reduced number of uterine implantation sites per litter, and reduced gestational
body weights were observed. Systemic exposure (AUC0-24 h) to amprenavir in rats was approximately 2 times the exposures in humans at the MRHD of fosamprenavir alone or approximately the same as those seen in humans at the MRHD of fosamprenavir in combination with ritonavir.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommends that HIV-1–infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection.
There is no information available on the presence of amprenavir in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. When administered to lactating rats, amprenavir was present in milk (see Data). Because of the potential for (1) HIV-1 transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive infants), and (3) adverse reactions in a breastfed infant, instruct mothers not to breastfeed if they are receiving fosamprenavir.
8.3 Females and Males of Reproductive Potential
Contraception
Use of fosamprenavir may reduce the efficacy of combined hormonal contraceptives. Advise patients using combined hormonal contraceptives to use an effective alternative contraceptive method or an additional barrier method of contraception [see Drug Interactions (7.2)].
8.4 Pediatric Use
The safety, pharmacokinetic profile, and virologic and immunologic responses of fosamprenavir with and without ritonavir were evaluated in protease inhibitor-naive and -experienced HIV-1–infected pediatric subjects aged at least 4 weeks to younger than 18 years and weighing at least 3 kg in 3 open-label trials [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.3)].
Treatment with fosamprenavir is not recommended in protease inhibitor-experienced pediatric patients younger than 6 months. The pharmacokinetics, safety, tolerability, and efficacy of fosamprenavir in pediatric patients younger than 4 weeks have not been established [see Clinical Pharmacology (12.3)]. Available pharmacokinetic and clinical data do not support once-daily dosing of fosamprenavir alone or in combination with ritonavir for any pediatrics or twice-daily dosing without ritonavir in pediatric patients younger than 2 years [see Clinical Pharmacology (12.3), Clinical Studies (14.3)]. See Dosage and Administration (2.3) for dosing recommendations for pediatric patients.
8.5 Geriatric Use
Clinical studies of fosamprenavir did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger adults. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Amprenavir is principally metabolized by the liver; therefore, caution should be exercised when administering fosamprenavir to patients with hepatic impairment because amprenavir concentrations may be increased [see Clinical Pharmacology (12.3)]. Patients with impaired hepatic function receiving fosamprenavir with or without concurrent ritonavir require dose reduction [see Dosage and Administration (2.4)].
There is no data to support dosing recommendations for pediatric subjects with hepatic impairment.
-
10 OVERDOSAGE
In a healthy volunteer repeat-dose pharmacokinetic trial evaluating high-dose combinations of fosamprenavir plus ritonavir, an increased frequency of Grade 2/3 ALT elevations (greater than 2.5 x ULN) was observed with fosamprenavir 1,400 mg twice daily plus ritonavir 200 mg twice daily (4 of 25 subjects). Concurrent Grade 1/2 elevations in AST (greater than 1.25 x ULN) were noted in 3 of these 4 subjects. These transaminase elevations resolved following discontinuation of dosing.
There is no known antidote for fosamprenavir. It is not known whether amprenavir can be removed by peritoneal dialysis or hemodialysis, although it is unlikely as amprenavir is highly protein bound. If overdosage occurs, the patient should be monitored for evidence of toxicity and standard supportive treatment applied as necessary.
-
11 DESCRIPTION
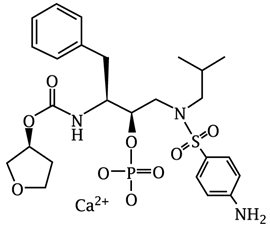
Fosamprenavir calcium is a prodrug of amprenavir, an inhibitor of HIV protease. The chemical name of fosamprenavir calcium is (3S)-tetrahydrofuran-3-yl (1S,2R)-3-[[(4-aminophenyl) sulfonyl](isobutyl)amino]-1-benzyl-2-(phosphonooxy) propylcarbamate monocalcium salt. Fosamprenavir calcium is a single stereoisomer with the (3S)(1S,2R) configuration. It has a molecular formula of C25H34CaN3O9PS and a molecular weight of 623.67. It has the following structural formula:
Fosamprenavir calcium is a white to cream color powder with a solubility of approximately 0.31 mg per mL in water at 25°C.
Fosamprenavir calcium tablets are available for oral administration in a strength of 700 mg of fosamprenavir as fosamprenavir calcium (equivalent to approximately 600 mg of amprenavir). Each 700-mg tablet contains the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, red iron oxide, silicified microcrystalline cellulose, titanium dioxide and triacetin.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
The pharmacokinetic properties of amprenavir after administration of fosamprenavir, with or without ritonavir, have been evaluated in both healthy adult volunteers and in HIV-1–infected subjects; no substantial differences in steady-state amprenavir concentrations were observed between the 2 populations.
The pharmacokinetic parameters of amprenavir after administration of fosamprenavir (with and without concomitant ritonavir) are shown in Table 7.
Table 7. Geometric Mean (95% CI) Steady-State Plasma Amprenavir Pharmacokinetic Parameters in Adults - * Data shown are median (range).
- † Ctau is the concentration at the end of the dose interval.
- ‡ HIV-infected adults.
- § AUC24 was calculated from AUC12 summary data x 2.
- ¶ Healthy adults.
Regimen
Cmax
(mcg/mL)
Tmax
(hours)*
AUC24
(mcgh/mL)
Ctau†
(mcg/mL)
Fosamprenavir
1,400 mg Twice Daily
(n = 22)‡
4.82
(4.06-5.72)
1.3
(0.8-4.0)
33.0§
(27.6-39.2)
0.35
(0.27-0.46)
Fosamprenavir
1,400 mg Once Daily plus Ritonavir 200 mg Once Daily
(n = 22)¶
7.24
(6.32-8.28)
2.1
(0.8-5.0)
69.4
(59.7-80.8)
1.45
(1.16-1.81)
Fosamprenavir
1,400 mg Once Daily plus Ritonavir 100 mg Once Daily
(n = 36)¶
7.93
(7.25-8.68)
1.5
(0.75-5.0)
66.4
(61.1-72.1)
0.86
(0.74-1.01)
Fosamprenavir
700 mg Twice Daily plus Ritonavir 100 mg Twice Daily
(n = 24)¶
6.08
(5.38-6.86)
1.5
(0.75-5.0)
79.2
(69.0-90.6)
2.12
(1.77-2.54)
The mean plasma amprenavir concentrations of the dosing regimens over the dosing intervals are displayed in Figure 1.
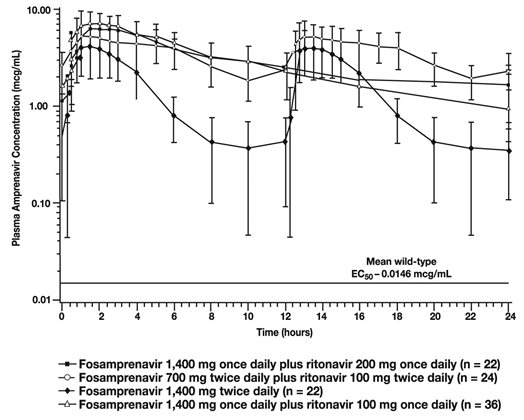
Figure 1. Mean (±SD) Steady-State Plasma Amprenavir Concentrations and Mean EC50 Values Against HIV from Protease Inhibitor-Naive Subjects (in the Absence of Human Serum)
Absorption
After administration of a single dose of fosamprenavir to HIV-1–infected subjects, the time to peak amprenavir concentration (Tmax) occurred between 1.5 and 4 hours (median 2.5 hours). The absolute oral bioavailability of amprenavir after administration of fosamprenavir in humans has not been established.
After administration of a single 1,400-mg dose in the fasted state, fosamprenavir calcium oral suspension (50 mg per mL) and fosamprenavir calcium tablets (700 mg) provided similar amprenavir exposures (AUC); however, the Cmax of amprenavir after administration of the suspension formulation was 14.5% higher compared with the tablet.
Amprenavir is both a substrate for and inducer of P-glycoprotein.
Effects of Food on Oral Absorption
Administration of a single 1,400 mg-dose of fosamprenavir calcium tablets in the fed state (standardized high-fat meal: 967 kcal, 67 grams fat, 33 grams protein, 58 grams carbohydrate) compared with the fasted state was associated with no significant changes in amprenavir Cmax, Tmax, or AUC0-∞ [see Dosage and Administration (2)].
Distribution
In vitro, amprenavir is approximately 90% bound to plasma proteins, primarily to alpha1-acid glycoprotein. In vitro, concentration-dependent binding was observed over the concentration range of 1 to 10 mcg per mL, with decreased binding at higher concentrations. The partitioning of amprenavir into erythrocytes is low, but increases as amprenavir concentrations increase, reflecting the higher amount of unbound drug at higher concentrations.
Metabolism
After oral administration, fosamprenavir is rapidly and almost completely hydrolyzed to amprenavir and inorganic phosphate prior to reaching the systemic circulation. This occurs in the gut epithelium during absorption. Amprenavir is metabolized in the liver by the CYP3A4 enzyme system. The 2 major metabolites result from oxidation of the tetrahydrofuran and aniline moieties. Glucuronide conjugates of oxidized metabolites have been identified as minor metabolites in urine and feces.
Elimination
Excretion of unchanged amprenavir in urine and feces is minimal. Unchanged amprenavir in urine accounts for approximately 1% of the dose; unchanged amprenavir was not detectable in feces. Approximately 14% and 75% of an administered single dose of 14C-amprenavir can be accounted for as metabolites in urine and feces, respectively. Two metabolites accounted for greater than 90% of the radiocarbon in fecal samples. The plasma elimination half-life of amprenavir is approximately 7.7 hours.
Specific Populations
Patients with Hepatic Impairment
The pharmacokinetics of amprenavir have been studied after the administration of fosamprenavir in combination with ritonavir to adult HIV-1–infected subjects with mild, moderate, and severe hepatic impairment. Following 2 weeks of dosing with fosamprenavir plus ritonavir, the AUC of amprenavir was increased by approximately 22% in subjects with mild hepatic impairment, by approximately 70% in subjects with moderate hepatic impairment, and by approximately 80% in subjects with severe hepatic impairment compared with HIV-1–infected subjects with normal hepatic function. Protein binding of amprenavir was decreased in subjects with hepatic impairment. The unbound fraction at 2 hours (approximate Cmax) ranged between a decrease of -7% to an increase of 57% while the unbound fraction at the end of the dosing interval (Cmin) increased from 50% to 102% [see Dosage and Administration (2.4)].
The pharmacokinetics of amprenavir have been studied after administration of amprenavir given as AGENERASE® capsules to adult subjects with hepatic impairment. Following administration of a single 600-mg oral dose, the AUC of amprenavir was increased by approximately 2.5-fold in subjects with moderate cirrhosis and by approximately 4.5-fold in subjects with severe cirrhosis compared with healthy volunteers [see Dosage and Administration (2.4)].
Patients with Renal Impairment
The impact of renal impairment on amprenavir elimination in adults has not been studied. The renal elimination of unchanged amprenavir represents approximately 1% of the administered dose; therefore, renal impairment is not expected to significantly impact the elimination of amprenavir.
Pregnant Women
Amprenavir pharmacokinetics were studied in pregnant women receiving fosamprenavir (700 mg) plus ritonavir (100 mg) twice daily during the second trimester (n = 6) or third trimester (n = 9) and postpartum (n = 9). Compared to postpartum, geometric mean amprenavir AUC was 35% lower in the second trimester and 25% lower in the third trimester (Table 8). This decrease results in amprenavir concentrations that are within the range observed across regimens of fosamprenavir in non-pregnant adults and lower concentrations compared with fosamprenavir (700 mg) plus ritonavir (100 mg) twice daily in non-pregnant adults (Table 7, Table 8). This decrease is not expected to be considered clinically relevant in patients who are virologically suppressed; however, viral load should be monitored closely to ensure viral suppression is maintained [see Dosage and Administration (2.2), Use in Specific Populations (8.1)]. The amprenavir geometric mean ratio (95% CI) of fetal cord to maternal peripheral plasma concentration (n = 7) was 0.27 (0.24 to 0.30).
Table 8. Geometric Mean (95% CI) Steady-State Plasma Amprenavir Pharmacokinetic Parameters in Pregnant Women Receiving Fosamprenavir with Ritonavir - * AUC24 was calculated from AUC12 summary data x 2.
- † Ctau represents the concentration at the end of the dose interval.
Pharmacokinetic
Parameter
Fosamprenavir 700 mg Twice Daily plus Ritonavir 100 mg Twice Daily
Second Trimester
(n = 6)
Third Trimester
(n = 9)
Postpartum
(n = 9)
AUC12 (mcgh/mL)
26.0
(19.5, 34.6)
30.1
(21.6, 41.9)
39.9
(31.9, 50.1)
AUC24 (mcgh/mL)*
52.0
(39.0, 69.2)
60.2
(43.2, 83.8)
79.8
(63.8, 100.2)
Cmax (mcg/mL)
4.19
(3.19, 5.51)
5.36
(3.98, 7.22)
6.65
(5.24, 8.44)
Ctau (mcg/mL)†
1.31
(0.97, 1.77)
1.34
(0.95, 1.89)
2.03
(1.46, 2.83)
Pediatric Patients
The pharmacokinetics of amprenavir following administration of fosamprenavir calcium oral suspension and fosamprenavir calcium tablets, with or without ritonavir, have been studied in a total of 212 HIV-1–infected pediatric subjects enrolled in 3 trials. Fosamprenavir without ritonavir was administered as 30 or 40 mg per kg twice daily to children aged 2 to 5 years. Fosamprenavir with ritonavir was administered as fosamprenavir 30 mg per kg plus ritonavir 6 mg per kg once daily to children aged 2 to 18 years and as fosamprenavir 18 to 60 mg per kg plus ritonavir 3 to 10 mg per kg twice daily to children aged at least 4 weeks to 18 years; body weights ranged from 3 to 103 kg.
Amprenavir apparent clearance decreased with increasing weight. Weight-adjusted apparent clearance was higher in children younger than 4 years, suggesting that younger children require higher mg-per-kg dosing of fosamprenavir.
The pharmacokinetics of fosamprenavir calcium oral suspension in protease inhibitor-naive infants younger than 6 months (n = 9) receiving fosamprenavir 45 mg per kg plus ritonavir 10 mg per kg twice daily generally demonstrated lower AUC12 and Cmin than adults receiving twice-daily fosamprenavir 700 mg plus ritonavir 100 mg, the dose recommended for protease-experienced adults. The mean steady-state amprenavir AUC12, Cmax, and Cmin were 26.6 mcghour per mL, 6.25 mcg per mL, and 0.86 mcg per mL, respectively. Because of expected low amprenavir exposure and a requirement for large volume of drug, twice-daily dosing of fosamprenavir alone (without ritonavir) in pediatric subjects younger than 2 years was not studied.
Pharmacokinetic parameters for fosamprenavir administered with food and with ritonavir in this patient population at the recommended weight-band-based dosage regimens are provided in Table 9.
Table 9. Geometric Mean (95% CI) Steady-State Plasma Amprenavir Pharmacokinetic Parameters by Weight in Pediatric and Adolescent Subjects Aged at Least 4 Weeks to 18 Years Receiving Fosamprenavir with Ritonavir - * Recommended dose for pediatric patients weighing 11 kg to less than 15 kg is based on population pharmacokinetic analysis.
Weight
Recommended Dosage Regimen
Cmax
AUC24
Cmin
n
(mcg/mL)
n
(mcgh/mL)
n
(mcg/mL)
< 11 kg
Fosamprenavir 45 mg/kg plus Ritonavir 7 mg/kg twice daily
12
6.00
(3.88, 9.29)
12
57.3
(34.1, 96.2)
27
1.65
(1.22, 2.24)
11 kg - < 15 kg
Fosamprenavir 30 mg/kg plus Ritonavir 3 mg/kg twice daily
Not studied*
15 kg - < 20 kg
Fosamprenavir 23 mg/kg plus Ritonavir 3 mg/kg twice daily
5
9.54
(4.63, 19.7)
5
121
(54.2, 269)
9
3.56
(2.33, 5.43)
20 kg - < 39 kg
Fosamprenavir 18 mg/kg plus Ritonavir 3 mg/kg twice daily
13
6.24
(5.01, 7.77)
12
97.9
(77.0, 124)
23
2.54
(2.11, 3.06)
≥ 39 kg
Fosamprenavir 700 mg plus Ritonavir 100 mg twice daily
15
5.03
(4.04, 6.26)
15
72.3
(59.6, 87.6)
42
1.98
(1.72, 2.29)
Subjects aged 2 to younger than 6 years receiving fosamprenavir 30 mg per kg twice daily without ritonavir achieved geometric mean (95% CI) amprenavir Cmax (n = 9), AUC12 (n = 9), and Cmin (n = 19) of 7.15 (5.05, 10.1), 22.3 (15.3, 32.6), and 0.513 (0.384, 0.686), respectively.
Geriatric Patients
The pharmacokinetics of amprenavir after administration of fosamprenavir to patients older than 65 years have not been studied [see Use in Specific Populations (8.5)].
Drug Interaction Studies
[See Contraindications (4), Warnings and Precautions (5.1), Drug Interactions (7).]
Amprenavir, the active metabolite of fosamprenavir, is metabolized in the liver by the cytochrome P450 enzyme system. Amprenavir inhibits CYP3A4. Data also suggest that amprenavir induces CYP3A4. Caution should be used when coadministering medications that are substrates, inhibitors, or inducers of CYP3A4, or potentially toxic medications that are metabolized by CYP3A4. Amprenavir does not inhibit CYP2D6, CYP1A2, CYP2C9, CYP2C19, CYP2E1, or uridine glucuronosyltransferase (UDPGT). Amprenavir is both a substrate for and inducer of P-glycoprotein.
Drug interaction trials were performed with fosamprenavir and other drugs likely to be coadministered or drugs commonly used as probes for pharmacokinetic interactions. The effects of coadministration on AUC, Cmax, and Cmin values are summarized in Table 10 (effect of other drugs on amprenavir) and Table 12 (effect of fosamprenavir on other drugs). In addition, since fosamprenavir delivers comparable amprenavir plasma concentrations as AGENERASE, drug interaction data derived from trials with AGENERASE are provided in Tables 11 and 13. For information regarding clinical recommendations, [see Drug Interactions (7)].
Table 10. Drug Interactions: Pharmacokinetic Parameters for Amprenavir after Administration of Fosamprenavir in the Presence of the Coadministered Drug(s) ↑ = Increase; ↓ = Decrease; ↔ = No change (↑ or ↓ less than or equal to 10%), NA = Not applicable. - * Concomitant medication is also shown in this column where appropriate.
- † Ritonavir Cmax, AUC, and Cmin increased by 63%, 45%, and 13%, respectively, compared with historical control.
- ‡ Compared with historical control.
- § Subjects were receiving fosamprenavir/ritonavir for 10 days prior to the 4-day treatment period with both ketoconazole and fosamprenavir/ritonavir.
- ¶ Compared with fosamprenavir 700 mg/ritonavir 100 mg twice daily for 2 weeks.
- # Subjects were receiving nevirapine for at least 12 weeks prior to trial.
- Þ Clast (C12 h or C24 h).
- ß Doses of fosamprenavir and raltegravir were given with food on pharmacokinetic sampling days and without regard to food all other days.
- à Compared with parallel control group.
Coadministered Drug(s) and Dose(s)
Dose of Fosamprenavir*
n
% Change in Amprenavir Pharmacokinetic Parameters (90% CI)
Cmax
AUC
Cmin
Antacid (MAALOX TC)
30-mL single dose
1,400-mg single dose
30
↓35
(↓24 to ↓42)
↓18
(↓9 to ↓26)
↑14
(↓7 to ↑39)
Atazanavir
300 mg once daily for 10 days
700 mg twice daily plus ritonavir 100 mg twice daily for 10 days
22
↔
↔
↔
Atorvastatin
10 mg once daily for 4 days
1,400 mg twice daily for 2 weeks
16
↓18
(↓34 to ↑1)
↓27
(↓41 to ↓12)
↓12
(↓27 to ↓6)
Atorvastatin
10 mg once daily for 4 days
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
16
↔
↔
↔
Efavirenz
600 mg once daily for 2 weeks
1,400 mg once daily plus ritonavir 200 mg once daily for 2 weeks
16
↔
↓13
(↓30 to ↑7)
↓36
(↓8 to ↓56)
Efavirenz
600 mg once daily plus additional ritonavir 100 mg once daily for 2 weeks
1,400 mg once daily plus ritonavir 200 mg once daily for 2 weeks
16
↑18
(↑1 to ↑38)
↑11
(0 to ↑24)
↔
Efavirenz
600 mg once daily for 2 weeks
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
16
↔
↔
↓17
(↓4 to ↓29)
Esomeprazole
20 mg once daily for 2 weeks
1,400 mg twice daily for 2 weeks
25
↔
↔
↔
Esomeprazole
20 mg once daily for 2 weeks
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
23
↔
↔
↔
Ethinyl estradiol/
norethindrone
0.035 mg/0.5 mg once daily for 21 days
700 mg twice daily plus ritonavir† 100 mg twice daily for 21 days
25
↔‡
↔‡
↔‡
Ketoconazole§
200 mg once daily for 4 days
700 mg twice daily plus ritonavir 100 mg twice daily for 4 days
15
↔
↔
↔
Lopinavir/ritonavir
533 mg/133 mg twice daily
1,400 mg twice daily for 2 weeks
18
↓13¶
↓26¶
↓42¶
Lopinavir/ritonavir
400 mg/100 mg twice daily for 2 weeks
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
18
↓58
(↓42 to ↓70)
↓63
(↓51 to ↓72)
↓65
(↓54 to ↓73)
Maraviroc
300 mg twice daily for 10 days
700 mg twice daily plus ritonavir 100 mg twice daily for 20 days
14
↓34
(↓25 to ↓41)
↓35
(↓29 to ↓41)
↓36
(↓27 to ↓43)
Maraviroc
300 mg once daily for 10 days
1,400 mg once daily plus ritonavir 100 mg once daily for 20 days
14
↓29
(↓20 to ↓38)
↓30
(↓23 to ↓36)
↓15
(↓3 to ↓25)
Methadone
70 to 120 mg once daily for 2 weeks
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
19
↔‡
↔‡
↔‡
Nevirapine
200 mg twice daily for 2 weeks#
1,400 mg twice daily for 2 weeks
17
↓25
(↓37 to ↓10)
↓33
(↓45 to ↓20)
↓35
(↓50 to ↓15)
Nevirapine
200 mg twice daily for 2 weeks#
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
17
↔
↓11
(↓23 to ↑3)
↓19
(↓32 to ↓4)
Phenytoin
300 mg once daily for 10 days
700 mg twice daily plus ritonavir 100 mg twice daily for 10 days
13
↔
↑20
(↑8 to ↑34)
↑19
(↑6 to ↑33)
Raltegravir
400 mg twice daily for 14 days
1,400 mg twice daily for 14 days (fasted)
14
↓27
(↓46 to ↔)
↓36
(↓53 to ↓13)
↓43Þ
(↓59 to ↓21)
1,400 mg twice daily for 14 daysß
14
↓15
(↓27 to ↓1)
↓17
(↓27 to ↓6)
↓32Þ
(↓53 to ↓1)
700 mg twice daily plus ritonavir 100 mg twice daily for 14 days (fasted)
14
↓14
(↓39 to ↑20)
↓17
(↓38 to ↑12)
↓20Þ
(↓45 to ↑17)
700 mg twice daily plus ritonavir 100 mg twice daily for 14 daysß
12
↓25
(↓42 to ↓2)
↓25
(↓44 to ↔)
↓33Þ
(↓52 to ↓7)
Raltegravir
400 mg twice daily for 14 days
1,400 mg once daily plus ritonavir 100 mg once daily for 14 days (fasted)
13
↓18
(↓34 to ↔)
↓24
(↓41 to ↔)
↓50Þ
(↓64 to ↓31)
1,400 mg once daily plus ritonavir 100 mg once daily for 14 daysß
14
↑27
(↓1 to ↑62)
↑13
(↓7 to ↑38)
↓17Þ
(↓45 to ↑26)
Ranitidine
300-mg single dose (administered 1 hour before fosamprenavir)
1,400-mg single dose
30
↓51
(↓43 to ↓58)
↓30
(↓22 to ↓37)
↔
(↓19 to ↑21)
Rifabutin
150 mg every other day for 2 weeks
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
15
↑36‡
(↑18 to ↑55)
↑35‡
(↑17 to ↑56)
↑17‡
(↓1 to ↑39)
Tenofovir
300 mg once daily for 4 to 48 weeks
700 mg twice daily plus ritonavir 100 mg twice daily for 4 to 48 weeks
45
NA
NA
↔à
Tenofovir
300 mg once daily for 4 to 48 weeks
1,400 mg once daily plus ritonavir 200 mg once daily for 4 to 48 weeks
60
NA
NA
↔à
Table 11. Drug Interactions: Pharmacokinetic Parameters for Amprenavir after Administration of AGENERASE in the Presence of the Coadministered Drug(s) ↑ = Increase; ↓ = Decrease; ↔ = No change (↑ or ↓ less than 10%); NA = Cmin not calculated for single-dose trial. - * Compared with parallel control group.
- † Median percent change; confidence interval not reported.
- ‡ Compared with historical data.
Coadministered Drug(s) and Dose(s)
Dose of AGENERASE*
n
% Change in Amprenavir Pharmacokinetic Parameters (90% CI)
Cmax
AUC
Cmin
Abacavir
300 mg twice daily for 2 to 3 weeks
900 mg twice daily for 2 to 3 weeks
4
↔*
↔*
↔*
Clarithromycin
500 mg twice daily for 4 days
1,200 mg twice daily for 4 days
12
↑15
(↑1 to ↑31)
↑18
(↑8 to ↑29)
↑39
(↑31 to ↑47)
Delavirdine
600 mg twice daily for 10 days
600 mg twice daily for 10 days
9
↑40†
↑130†
↑125†
Ethinyl estradiol/
norethindrone
0.035 mg/1 mg for 1 cycle
1,200 mg twice daily for 28 days
10
↔
↓22
(↓35 to ↓8)
↓20
(↓41 to ↑8)
Indinavir
800 mg 3 times a day for 2 weeks (fasted)
750 or 800 mg 3 times a day for 2 weeks (fasted)
9
↑18
(↑13 to ↑58)
↑33
(↑2 to ↑73)
↑25
(↓27 to ↑116)
Ketoconazole
400-mg single dose
1,200-mg single dose
12
↓16
(↓25 to ↓6)
↑31
(↑20 to ↑42)
NA
Lamivudine
150-mg single dose
600-mg single dose
11
↔
↔
NA
Methadone
44 to 100 mg once daily for
> 30 days
1,200 mg twice daily for 10 days
16
↓27‡
↓30‡
↓25‡
Nelfinavir
750 mg 3 times a day for 2 weeks (fed)
750 or 800 mg 3 times a day for 2 weeks (fed)
6
↓14
(↓38 to ↑20)
↔
↑189
(↑52 to ↑448)
Rifabutin
300 mg once daily for 10 days
1,200 mg twice daily for 10 days
5
↔
↓15
(↓28 to 0)
↓15
(↓38 to ↑17)
Rifampin
300 mg once daily for 4 days
1,200 mg twice daily for 4 days
11
↓70
(↓76 to ↓62)
↓82
(↓84 to ↓78)
↓92
(↓95 to ↓89)
Saquinavir
800 mg 3 times a day for 2 weeks (fed)
750 or 800 mg 3 times a day for 2 weeks (fed)
7
↓37
(↓54 to ↓14)
↓32
(↓49 to ↓9)
↓14
(↓52 to ↑54)
Zidovudine
300-mg single dose
600-mg single dose
12
↔
↑13
(↓2 to ↑31)
NA
Table 12. Drug Interactions: Pharmacokinetic Parameters for Coadministered Drug in the Presence of Amprenavir after Administration of Fosamprenavir ↑ = Increase; ↓ = Decrease; ↔ = No change (↑ or ↓less than 10%); ND = Interaction cannot be determined as Cmin was below the lower limit of quantitation. - * Concomitant medication is also shown in this column where appropriate.
- † Comparison arm of atazanavir 300 mg once daily plus ritonavir 100 mg once daily for 10 days.
- ‡ Administered as a combination oral contraceptive tablet: ethinyl estradiol 0.035 mg/norethindrone 0.5 mg.
- § Subjects were receiving fosamprenavir/ritonavir for 10 days prior to the 4-day treatment period with both ketoconazole and fosamprenavir/ritonavir.
- ¶ Data represent lopinavir concentrations.
- # Compared with lopinavir 400 mg/ritonavir 100 mg twice daily for 2 weeks.
- Þ Dose normalized to methadone 100 mg. The unbound concentration of the active moiety, R-methadone, was unchanged.
- ß Subjects were receiving nevirapine for at least 12 weeks prior to trial.
- à Comparison arm of rifabutin 300 mg once daily for 2 weeks. AUC is AUC(0-48 h).
Coadministered Drug(s) and Dose(s)
Dose of Fosamprenavir*
n
% Change in Pharmacokinetic Parameters of Coadministered Drug (90% CI)
Cmax
AUC
Cmin
Atazanavir
300 mg once daily for 10 days†
700 mg twice daily plus ritonavir 100 mg twice daily for 10 days
21
↓24
(↓39 to ↓6)
↓22
(↓34 to ↓9)
↔
Atorvastatin
10 mg once daily for 4 days
1,400 mg twice daily for 2 weeks
16
↑304
(↑205 to ↑437)
↑130
(↑100 to ↑164)
↓10
(↓27 to ↑12)
Atorvastatin
10 mg once daily for 4 days
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
16
↑184
(↑126 to ↑257)
↑153
(↑115 to ↑199)
↑73
(↑45 to ↑108)
Esomeprazole
20 mg once daily for 2 weeks
1,400 mg twice daily for 2 weeks
25
↔
↑55
(↑39 to ↑73)
ND
Esomeprazole
20 mg once daily for 2 weeks
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
23
↔
↔
ND
Ethinyl estradiol‡
0.035 mg once daily for 21 days
700 mg twice daily plus ritonavir 100 mg twice daily for 21 days
25
↓28
(↓21 to ↓35)
↓37
(↓30 to ↓42)
ND
Dolutegravir
50 mg once daily
700 mg twice daily plus ritonavir 100 mg twice daily
12
↓24
(↓8 to ↓37)
↓35
(↓22 to ↓46)
↓49
(↓37 to ↓59)
Ketoconazole§
200 mg once daily for 4 days
700 mg twice daily plus ritonavir 100 mg twice daily for 4 days
15
↑25
(↑0 to ↑56)
↑169
(↑108 to ↑248)
ND
Lopinavir/ritonavir¶
533 mg/133 mg twice daily for 2 weeks
1,400 mg twice daily for 2 weeks
18
↔#
↔#
↔#
Lopinavir/ritonavir¶
400 mg/100 mg twice daily for 2 weeks
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
18
↑30
(↓15 to ↑47)
↑37
(↓20 to ↑55)
↑52
(↓28 to ↑82)
Maraviroc
300 mg twice daily for 10 days
700 mg twice daily plus ritonavir 100 mg twice daily for 20 days
14
↑52
(↑27 to ↑82)
↑149
(↑119 to ↑182)
↑374
(↑303 to ↑457)
Maraviroc
300 mg once daily for 10 days
1,400 mg once daily plus ritonavir 100 mg once daily for 20 days
14
↑45
(↑20 to ↑74)
↑126
(↑99 to ↑158)
↑80
(↑53 to ↑113)
Methadone
70 to 120 mg once daily for 2 weeks
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
19
R-Methadone (active)
↓21Þ
(↓30 to ↓12)
↓18Þ
(↓27 to ↓8)
↓11Þ
(↓21 to ↑1)
S-Methadone (inactive)
↓43Þ
(↓49 to ↓37)
↓43Þ
(↓50 to ↓36)
↓41Þ
(↓49 to ↓31)
Nevirapine
200 mg twice daily for 2 weeksß
1,400 mg twice daily for 2 weeks
17
↑25
(↑14 to ↑37)
↑29
(↑19 to ↑40)
↑34
(↑20 to ↑49)
Nevirapine
200 mg twice daily for 2 weeksß
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
17
↑13
(↑3 to ↑24)
↑14
(↑5 to ↑24)
↑22
(↑9 to ↑35)
Norethindrone‡
0.5 mg once daily for 21 days
700 mg twice daily plus ritonavir 100 mg twice daily for 21 days
25
↓38
(↓32 to ↓44)
↓34
(↓30 to ↓37)
↓26
(↓20 to ↓32)
Phenytoin
300 mg once daily for 10 days
700 mg twice daily plus ritonavir 100 mg twice daily for 10 days
14
↓20
(↓12 to ↓27)
↓22
(↓17 to ↓27)
↓29
(↓23 to ↓34)
Rifabutin
150 mg every other day for 2 weeksà
700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks
15
↓14
(↓28 to ↑4)
↔
↑28
(↑12 to ↑46)
(25-O-desacetylrifabutin metabolite)
↑579
(↑479 to ↑698)
↑1,120
(↑965 to ↑1,300)
↑2,510
(↑1,910 to ↑3,300)
Rifabutin + 25-O-desacetylrifabutin metabolite
NA
↑64
(↑46 to ↑84)
NA
Rosuvastatin
10-mg single dose
700 mg twice daily plus ritonavir 100 mg twice daily for 7 days
↑45
↑8
NA
Table 13. Drug Interactions: Pharmacokinetic Parameters for Coadministered Drug in the Presence of Amprenavir after Administration of AGENERASE
↑ = Increase; ↓ = Decrease; ↔ = No change (↑ or ↓ less than 10%); NA = Cmin not calculated for single-dose trial; ND = Interaction cannot be determined as Cmin was below the lower limit of quantitation. - * Compared with historical data.
- † Median percent change; confidence interval not reported.
Coadministered Drug(s) and Dose(s)
Dose of AGENERASE
n
% Change in Pharmacokinetic Parameters of Coadministered Drug (90% CI)
Cmax
AUC
Cmin
Abacavir
300 mg twice daily for 2 to 3 weeks
900 mg twice daily for 2 to 3 weeks
4
↔*
↔*
↔*
Clarithromycin
500 mg twice daily for 4 days
1,200 mg twice daily for 4 days
12
↓10
(↓24 to ↑7)
↔
↔
Delavirdine
600 mg twice daily for 10 days
600 mg twice daily for 10 days
9
↓47†
↓61†
↓88†
Ethinyl estradiol
0.035 mg for 1 cycle
1,200 mg twice daily for 28 days
10
↔
↔
↑32
(↓3 to ↑79)
Indinavir
800 mg 3 times a day for 2 weeks (fasted)
750 mg or 800 mg 3 times a day for 2 weeks (fasted)
9
↓22*
↓38*
↓27*
Ketoconazole
400-mg single dose
1,200-mg single dose
12
↑19
(↑8 to ↑33)
↑44
(↑31 to ↑59)
NA
Lamivudine
150-mg single dose
600-mg single dose
11
↔
↔
NA
Methadone
44 mg to 100 mg once daily for > 30 days
1,200 mg twice daily for 10 days
16
R-Methadone (active)
↓25
(↓32 to ↓18)
↓13
(↓21 to ↓5)
↓21
(↓32 to ↓9)
S-Methadone (inactive)
↓48
(↓55 to ↓40)
↓40
(↓46 to ↓32)
↓53
(↓60 to ↓43)
Nelfinavir
750 mg 3 times a day for 2 weeks (fed)
750 mg or 800 mg 3 times a day for 2 weeks (fed)
6
↑12*
↑15*
↑14*
Norethindrone
1 mg for 1 cycle
1,200 mg twice daily for 28 days
10
↔
↑18
(↑1 to ↑38)
↑45
(↑13 to ↑88)
Rifabutin
300 mg once daily for 10 days
1,200 mg twice daily for 10 days
5
↑119
(↑82 to ↑164)
↑193
(↑156 to ↑235)
↑271
(↑171 to ↑409)
Rifampin
300 mg once daily for 4 days
1,200 mg twice daily for 4 days
11
↔
↔
ND
Saquinavir
800 mg 3 times a day for 2 weeks (fed)
750 mg or 800 mg 3 times a day for 2 weeks (fed)
7
↑21*
↓19*
↓48*
Zidovudine
300-mg single dose
600-mg single dose
12
↑40
(↑14 to ↑71)
↑31
(↑19 to ↑45)
NA
12.4 Microbiology
Mechanism of Action
Fosamprenavir is a prodrug that is rapidly hydrolyzed to amprenavir by cellular phosphatases in the gut epithelium as it is absorbed. Amprenavir is an inhibitor of HIV-1 protease. Amprenavir binds to the active site of HIV-1 protease and thereby prevents the processing of viral Gag and Gag-Pol polyprotein precursors, resulting in the formation of immature non-infectious viral particles.
Antiviral Activity
Fosamprenavir has little or no antiviral activity in cell culture. The antiviral activity of amprenavir was evaluated against HIV-1 IIIB in both acutely and chronically infected lymphoblastic cell lines (MT-4, CEM-CCRF, H9) and in peripheral blood lymphocytes in cell culture. The 50% effective concentration (EC50) of amprenavir ranged from 0.012 to 0.08 microM in acutely infected cells and was 0.41 microM in chronically infected cells (1 microM = 0.50 mcg per mL). The median EC50 value of amprenavir against HIV-1 isolates from clades A to G was 0.00095 microM in peripheral blood mononuclear cells (PBMCs). Similarly, the EC50 values for amprenavir against monocytes/macrophage tropic HIV-1 isolates (clade B) ranged from 0.003 to 0.075 microM in monocyte/macrophage cultures. The EC50 values of amprenavir against HIV-2 isolates grown in PBMCs were higher than those for HIV-1 isolates, and ranged from 0.003 to 0.11 microM. The anti-HIV-1 activity of amprenavir was not antagonistic in combination with the nucleoside reverse transcriptase inhibitors (NRTIs); abacavir, didanosine, lamivudine, stavudine, tenofovir, and zidovudine; the non-nucleoside reverse transcriptase inhibitors (NNRTIs) delavirdine, efavirenz, and nevirapine; the protease inhibitors (PIs) atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, and saquinavir; and the gp41 fusion inhibitor enfuvirtide. These drug combinations have not been adequately studied in humans.
Resistance
HIV-1 isolates with decreased susceptibility to amprenavir have been selected in cell culture and obtained from subjects treated with fosamprenavir. Genotypic analysis of isolates from treatment-naive subjects failing amprenavir-containing regimens showed substitutions in the HIV-1 protease resulting in amino acid substitutions primarily at positions V32I, M46I/L, I47V, I50V, I54L/M, and I84V, as well as substitutions in the p7/p1 and p1/p6 Gag and Gag-Pol polyprotein precursor cleavage sites. Some of these amprenavir resistance-associated substitutions have also been detected in HIV-1 isolates from antiretroviral-naive subjects treated with fosamprenavir. Of the 488 antiretroviral-naive subjects treated with fosamprenavir 1,400 mg twice daily or fosamprenavir 1,400 mg plus ritonavir 200 mg once daily in Trials APV30001 and APV30002, respectively, isolates from 61 subjects (29 receiving fosamprenavir and 32 receiving fosamprenavir/ritonavir) with virologic failure (plasma HIV-1 RNA greater than 1,000 copies per mL on 2 occasions on or after Week 12) were genotyped. Isolates from 5 of the 29 antiretroviral-naive subjects (17%) receiving fosamprenavir without ritonavir in Trial APV30001 had evidence of genotypic resistance to amprenavir: I54L/M (n = 2), I54L + L33F (n = 1), V32I + I47V (n = 1), and M46I + I47V (n = 1). No amprenavir resistance-associated substitutions were detected in isolates from antiretroviral-naive subjects treated with fosamprenavir/ritonavir for 48 weeks in Trial APV30002. However, the M46I and I50V substitutions were detected in isolates from 1 virologic failure subject receiving fosamprenavir/ritonavir once daily at Week 160 (HIV-1 RNA greater than 500 copies per mL). Upon retrospective analysis of stored samples using an ultrasensitive assay, these resistant substitutions were traced back to Week 84 (76 weeks prior to clinical virologic failure).
Cross-Resistance
Varying degrees of cross-resistance among HIV-1 protease inhibitors have been observed. An association between virologic response at 48 weeks (HIV-1 RNA level less than 400 copies per mL) and protease inhibitor-resistance substitutions detected in baseline HIV-1 isolates from protease inhibitor-experienced subjects receiving fosamprenavir/ritonavir twice daily (n = 88), or lopinavir/ritonavir twice daily (n = 85) in Trial APV30003 is shown in Table 14. The majority of subjects had previously received either one (47%) or 2 protease inhibitors (36%), most commonly nelfinavir (57%) and indinavir (53%). Out of 102 subjects with baseline phenotypes receiving twice-daily fosamprenavir/ritonavir, 54% (n = 55) had resistance to at least one protease inhibitor, with 98% (n = 54) of those having resistance to nelfinavir. Out of 97 subjects with baseline phenotypes in the lopinavir/ritonavir arm, 60% (n = 58) had resistance to at least one protease inhibitor, with 97% (n = 56) of those having resistance to nelfinavir.
Table 14. Responders at Trial Week 48 by Presence of Baseline Protease Inhibitor Resistance-Associated Substitutions* - * Results should be interpreted with caution because the subgroups were small.
- † Most subjects had greater than 1 protease inhibitor resistance-associated substitution at baseline.
Protease Inhibitor
Resistance-Associated Substitutions†
Fosamprenavir/Ritonavir
Twice Daily(n = 88)
Lopinavir/Ritonavir
Twice Daily(n = 85)
D30N
21/22
95%
17/19
89%
N88D/S
20/22
91%
12/12
100%
L90M
16/31
52%
17/29
59%
M46I/L
11/22
50%
12/24
50%
V82A/F/T/S
2/9
22%
6/17
35%
I54V
2/11
18%
6/11
55%
I84V
1/6
17%
2/5
40%
The virologic response based upon baseline phenotype was assessed. Baseline isolates from protease inhibitor-experienced subjects responding to fosamprenavir/ritonavir twice daily had a median shift in susceptibility to amprenavir relative to a standard wild-type reference strain of 0.7 (range: 0.1 to 5.4, n = 62), and baseline isolates from individuals failing therapy had a median shift in susceptibility of 1.9 (range: 0.2 to 14, n = 29). Because this was a select patient population, these data do not constitute definitive clinical susceptibility break points. Additional data are needed to determine clinically relevant break points for fosamprenavir.
Isolates from 15 of the 20 subjects receiving twice-daily fosamprenavir/ritonavir up to Week 48 and experiencing virologic failure/ongoing replication were subjected to genotypic analysis. The following amprenavir resistance-associated substitutions were found either alone or in combination: V32I, M46I/L, I47V, I50V, I54L/M, and I84V. Isolates from 4 of the 16 subjects continuing to receive twice-daily fosamprenavir/ritonavir up to Week 96 who experienced virologic failure underwent genotypic analysis. Isolates from 2 subjects contained amprenavir resistance-associated substitutions: V32I, M46I, and I47V in 1 isolate and I84V in the other.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In long-term carcinogenicity studies, fosamprenavir was administered orally for up to 104 weeks at doses of 250, 400, or 600 mg per kg per day in mice and at doses of 300, 825, or 2,250 mg per kg per day in rats. Exposures at these doses were 0.3- to 0.7-fold (mice) and 0.7- to 1.4-fold (rats) those in humans given 1,400 mg twice daily of fosamprenavir alone, and 0.2- to 0.3-fold (mice) and 0.3- to 0.7-fold (rats) those in humans given 1,400 mg once daily of fosamprenavir plus 200 mg ritonavir once daily. Exposures in the carcinogenicity studies were 0.1- to 0.3-fold (mice) and 0.3- to 0.6-fold (rats) those in humans given 700 mg of fosamprenavir plus 100 mg ritonavir twice daily. There was an increase in hepatocellular adenomas and hepatocellular carcinomas at all doses in male mice and at 600 mg per kg per day in female mice, and in hepatocellular adenomas and thyroid follicular cell adenomas at all doses in male rats, and at 825 mg per kg per day and 2,250 mg per kg per day in female rats. The relevance of the hepatocellular findings in the rodents for humans is uncertain. Repeat-dose studies with fosamprenavir in rats produced effects consistent with enzyme induction, which predisposes rats, but not humans, to thyroid neoplasms. In addition, in rats only there was an increase in interstitial cell hyperplasia at 825 mg per kg per day and 2,250 mg per kg per day, and an increase in uterine endometrial adenocarcinoma at 2,250 mg per kg per day. The incidence of endometrial findings was slightly increased over concurrent controls, but was within background range for female rats. The relevance of the uterine endometrial adenocarcinoma findings in rats for humans is uncertain.
Fosamprenavir was not mutagenic or genotoxic in a battery of in vitro and in vivo assays. These assays included bacterial reverse mutation (Ames), mouse lymphoma, rat micronucleus, and chromosome aberrations in human lymphocytes.
The effects of fosamprenavir on fertility and general reproductive performance were investigated in male (treated for 4 weeks before mating) and female rats (treated for 2 weeks before mating through Postpartum Day 6) that received doses of 300, 820, or 2,240 mg per kg per day. Systemic exposures (AUC0-24 h) to amprenavir in these studies were 3 (males) to 4 (females) times higher than exposures in humans following administration of the MRHD of fosamprenavir alone or similar to those seen in humans following administration of fosamprenavir in combination with ritonavir. Fosamprenavir did not impair mating or fertility of male or female rats and did not affect the development and maturation of sperm from treated rats.
-
14 CLINICAL STUDIES
14.1 Therapy-Naive Adult Trials
APV30001
A randomized, open-label trial evaluated treatment with fosamprenavir calcium tablets (1,400 mg twice daily) versus nelfinavir (1,250 mg twice daily) in 249 antiretroviral treatment-naive subjects. Both groups of subjects also received abacavir (300 mg twice daily) and lamivudine (150 mg twice daily).
The mean age of the subjects in this trial was 37 years (range: 17 to 70 years); 69% of the subjects were male, 20% were CDC Class C (AIDS), 24% were white, 32% were black, and 44% were Hispanic. At baseline, the median CD4+ cell count was 212 cells per mm3 (range: 2 to 1,136 cells per mm3; 18% of subjects had a CD4+ cell count of less than 50 cells per mm3 and 30% were in the range of 50 to less than 200 cells per mm3). Baseline median HIV-1 RNA was 4.83 log10 copies per mL (range: 1.69 to 7.41 log10 copies per mL; 45% of subjects had greater than 100,000 copies per mL).
The outcomes of randomized treatment are provided in Table 15.
Table 15. Outcomes of Randomized Treatment through Week 48 (APV30001) - * Subjects achieved and maintained confirmed HIV-1 RNA less than 400 copies per mL (less than 50 copies per mL) through Week 48 (Roche AMPLICOR HIV-1 MONITOR Assay Version 1.5).
- † Includes consent withdrawn, lost to follow up, protocol violations, those with missing data, and other reasons.
Outcome
(Rebound or discontinuation = failure)
Fosamprenavir
1,400 mg
Twice Daily(n = 166)
Nelfinavir
1,250 mg
Twice Daily(n = 83)
Responder*
66% (57%)
52% (42%)
Virologic failure
19%
32%
Rebound
16%
19%
Never suppressed through Week 48
3%
13%
Clinical progression
1%
1%
Death
0%
1%
Discontinued due to adverse reactions
4%
2%
Discontinued due to other reasons†
10%
10%
Treatment response by viral load strata is shown in Table 16.
Table 16. Proportions of Responders through Week 48 by Screening Viral Load (APV30001) Screening Viral Load
HIV-1 RNA
(copies/mL)
Fosamprenavir
1,400 mg
Twice DailyNelfinavir
1,250 mg
Twice Daily< 400 copies/mL
n
< 400 copies/mL
n
≤ 100,000
65%
93
65%
46
> 100,000
67%
73
36%
37
Through 48 weeks of therapy, the median increases from baseline in CD4+ cell counts were 201 cells per mm3 in the group receiving fosamprenavir and 216 cells per mm3 in the nelfinavir group.
APV30002
A randomized, open-label trial evaluated treatment with fosamprenavir calcium tablets (1,400 mg once daily) plus ritonavir (200 mg once daily) versus nelfinavir (1,250 mg twice daily) in 649 treatment-naive subjects. Both treatment groups also received abacavir (300 mg twice daily) and lamivudine (150 mg twice daily).
The mean age of the subjects in this trial was 37 years (range: 18 to 69 years); 73% of the subjects were male, 22% were CDC Class C, 53% were white, 36% were black, and 8% were Hispanic. At baseline, the median CD4+ cell count was 170 cells per mm3 (range: 1 to 1,055 cells per mm3; 20% of subjects had a CD4+ cell count of less than 50 cells per mm3 and 35% were in the range of 50 to less than 200 cells per mm3). Baseline median HIV-1 RNA was 4.81 log10 copies per mL (range: 2.65 to 7.29 log10 copies per mL; 43% of subjects had greater than 100,000 copies per mL).
The outcomes of randomized treatment are provided in Table 17.
Table 17. Outcomes of Randomized Treatment through Week 48 (APV30002) - * Subjects achieved and maintained confirmed HIV-1 RNA less than 400 copies per mL (less than 50 copies per mL) through Week 48 (Roche AMPLICOR HIV-1 MONITOR Assay Version 1.5).
- † Includes consent withdrawn, lost to follow up, protocol violations, those with missing data, and other reasons.
Outcome
(Rebound or discontinuation = failure)
Fosamprenavir
1,400 mg/
Ritonavir 200 mg
Once Daily(n = 322)
Nelfinavir
1,250 mg
Twice Daily(n = 327)
Responder*
69% (58%)
68% (55%)
Virologic failure
6%
16%
Rebound
5%
8%
Never suppressed through Week 48
1%
8%
Death
1%
0%
Discontinued due to adverse reactions
9%
6%
Discontinued due to other reasons†
15%
10%
Treatment response by viral load strata is shown in Table 18.
Table 18. Proportions of Responders through Week 48 by Screening Viral Load (APV30002) Screening Viral Load
HIV-1 RNA
(copies/mL)
Fosamprenavir
1,400 mg/
Ritonavir 200 mg Once Daily
Nelfinavir
1,250 mg
Twice Daily< 400 copies/mL
n
< 400 copies/mL
n
≤ 100,000
72%
197
73%
194
> 100,000
66%
125
64%
133
Through 48 weeks of therapy, the median increases from baseline in CD4+ cell counts were 203 cells per mm3 in the group receiving fosamprenavir and 207 cells per mm3 in the nelfinavir group.
14.2 Protease Inhibitor-Experienced Adult Trials
APV30003
A randomized, open-label, multicenter trial evaluated 2 different regimens of fosamprenavir plus ritonavir (fosamprenavir calcium tablets 700 mg twice daily plus ritonavir 100 mg twice daily or fosamprenavir calcium tablets 1,400 mg once daily plus ritonavir 200 mg once daily) versus lopinavir/ritonavir (400 mg/100 mg twice daily) in 315 subjects who had experienced virologic failure to 1 or 2 prior protease inhibitor-containing regimens.
The mean age of the subjects in this trial was 42 years (range: 24 to 72 years); 85% were male, 33% were CDC Class C, 67% were white, 24% were black, and 9% were Hispanic. The median CD4+ cell count at baseline was 263 cells per mm3 (range: 2 to 1,171 cells per mm3). Baseline median plasma HIV-1 RNA level was 4.14 log10 copies per mL (range: 1.69 to 6.41 log10 copies per mL).
The median durations of prior exposure to NRTIs were 257 weeks for subjects receiving fosamprenavir/ritonavir twice daily (79% had greater than or equal to 3 prior NRTIs) and 210 weeks for subjects receiving lopinavir/ritonavir (64% had greater than or equal to 3 prior NRTIs). The median durations of prior exposure to protease inhibitors were 149 weeks for subjects receiving fosamprenavir/ritonavir twice daily (49% received greater than or equal to 2 prior protease inhibitors) and 130 weeks for subjects receiving lopinavir/ritonavir (40% received greater than or equal to 2 prior protease inhibitors).
The time-averaged changes in plasma HIV-1 RNA from baseline (AAUCMB) at 48 weeks (the endpoint on which the trial was powered) were -1.4 log10 copies per mL for twice-daily fosamprenavir/ritonavir and -1.67 log10 copies per mL for the lopinavir/ritonavir group.
The proportions of subjects who achieved and maintained confirmed HIV-1 RNA less than 400 copies per mL (secondary efficacy endpoint) were 58% with twice-daily fosamprenavir/ritonavir and 61% with lopinavir/ritonavir (95% CI for the difference: -16.6, 10.1). The proportions of subjects with HIV-1 RNA less than 50 copies per mL with twice-daily fosamprenavir/ritonavir and with lopinavir/ritonavir were 46% and 50%, respectively (95% CI for the difference: -18.3, 8.9). The proportions of subjects who were virologic failures were 29% with twice-daily fosamprenavir/ritonavir and 27% with lopinavir/ritonavir.
The frequency of discontinuations due to adverse events and other reasons, and deaths were similar between treatment arms.
Through 48 weeks of therapy, the median increases from baseline in CD4+ cell counts were 81 cells per mm3 with twice-daily fosamprenavir/ritonavir and 91 cells per mm3 with lopinavir/ritonavir.
This trial was not large enough to reach a definitive conclusion that fosamprenavir/ritonavir and lopinavir/ritonavir are clinically equivalent.
Once-daily administration of fosamprenavir plus ritonavir is not recommended for protease inhibitor-experienced patients. Through Week 48, 50% and 37% of subjects receiving fosamprenavir 1,400 mg plus ritonavir 200 mg once daily had plasma HIV-1 RNA less than 400 copies per mL and less than 50 copies per mL, respectively.
14.3 Pediatric Trials
Three open-label trials in pediatric subjects aged at least 4 weeks to 18 years were conducted. In one trial (APV29005), twice-daily dosing regimens (fosamprenavir with or without ritonavir) were evaluated in combination with other antiretroviral agents in pediatric subjects aged 2 to 18 years. In a second trial (APV20002), twice-daily dosing regimens (fosamprenavir with ritonavir) were evaluated in combination with other antiretroviral agents in pediatric subjects aged at least 4 weeks to younger than 2 years. A third trial (APV20003) evaluated once-daily dosing of fosamprenavir with ritonavir; the pharmacokinetic data from this trial did not support a once-daily dosing regimen in any pediatric patient population.
APV29005
Fosamprenavir
Twenty (18 therapy-naive and 2 therapy-experienced) pediatric subjects received fosamprenavir calcium oral suspension without ritonavir twice daily. At Week 24, 65% (13 of 20) achieved HIV-1 RNA less than 400 copies per mL, and the median increase from baseline in CD4+ cell count was 350 cells per mm3.
Fosamprenavir Plus Ritonavir
Forty-nine protease inhibitor-naive and 40 protease inhibitor-experienced pediatric subjects received fosamprenavir calcium oral suspension or tablets with ritonavir twice daily. At Week 24, 71% of protease inhibitor-naive (35 of 49) and 55% of protease inhibitor-experienced (22 of 40) subjects achieved HIV-1 RNA less than 400 copies per mL; median increases from baseline in CD4+ cell counts were 184 cells per mm3 and 150 cells per mm3 in protease inhibitor-naive and experienced subjects, respectively.
APV20002
Fifty-four pediatric subjects (49 protease inhibitor-naive and 5 protease inhibitor-experienced) received fosamprenavir calcium oral suspension with ritonavir twice daily. At Week 24, 72% of subjects achieved HIV-1 RNA less than 400 copies per mL. The median increases from baseline in CD4+ cell counts were 400 cells per mm3 in subjects aged at least 4 weeks to younger than 6 months and 278 cells per mm3 in subjects aged 6 months to 2 years.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Fosamprenavir Calcium Tablets are available containing 700 mg of fosamprenavir as fosamprenavir calcium.
The 700 mg tablets are pink, film-coated, modified capsule shaped, unscored tablets debossed with M on one side of the tablet and FT7 on the other side. They are available as follows:
NDC: 0378-3520-91
bottles of 60 tabletsStore at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Keep container tightly closed.
Dispense in original container.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Drug Interactions: A statement to patients and healthcare providers is included on the product’s bottle label: ALERT: Find out about medicines that should NOT be taken with Fosamprenavir Calcium Tablets.
Fosamprenavir calcium tablets may interact with many drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, particularly St. John’s wort.
Advise patients receiving PDE5 inhibitors that they may be at an increased risk of PDE5 inhibitor-associated adverse events, including hypotension, visual changes, and priapism, and should promptly report any symptoms to their healthcare provider [see Contraindications (4), Warnings and Precautions (5.1), Drug Interactions (7)].
Contraception:Instruct patients receiving combined hormonal contraception to use an effective alternative contraceptive method or an additional barrier method during therapy with fosamprenavir calcium tablets because hormonal levels may decrease, and if used in combination with fosamprenavir calcium tablets and ritonavir, liver enzyme elevations may occur [see Drug Interactions (7.2), Use in Specific Populations (8.3)].
Severe Skin Reactions: Advise patients that skin reactions ranging from mild to severe, including Stevens-Johnson Syndrome, have been reported with fosamprenavir calcium tablets. Advise patients to discontinue fosamprenavir calcium tablets immediately for severe or life-threatening skin reactions or for moderate rashes accompanied by systemic symptoms [see Warnings and Precautions (5.2), Adverse Reactions (6)].
Sulfa Allergy: Advise patients to inform their healthcare provider if they have a sulfa allergy. The potential for cross-sensitivity between drugs in the sulfonamide class and fosamprenavir is unknown [see Warnings and Precautions (5.3)].
Hepatic Toxicity: Advise patients that it is recommended to have laboratory testing before and during therapy as patients with underlying hepatitis B or C or marked elevations of transaminases prior to treatment may be at increased risk for developing or worsening transaminase elevations with use of fosamprenavir calcium tablets, particularly at higher than recommended doses which should not be used [see Warnings and Precautions (5.4)].
Immune Reconstitution Syndrome:Advise patients to inform their healthcare provider immediately of any signs or symptoms of infection as inflammation from previous infection may occur soon after combination antiretroviral therapy, including when fosamprenavir calcium tablets are started [see Warnings and Precautions (5.6)].
Increase in Body Fat:Inform patients that an increase of body fat may occur in patients receiving protease inhibitors, including fosamprenavir calcium tablets, and that the cause and long-term health effects of these conditions are not known at this time [see Warnings and Precautions (5.7)].
Lipid Elevations:Advise patients that it is recommended to have laboratory testing before and during therapy as increases in the concentration of triglycerides and cholesterol have been reported with use of fosamprenavir calcium tablets [see Warnings and Precautions (5.8), Adverse Reactions (6)].
Pregnancy Registry:Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to fosamprenavir calcium tablets during pregnancy [see Use in Specific Populations (8.1)].
Lactation: Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in the breast milk [see Use in Specific Populations (8.2)].
Missed Dose: Instruct patients that if they miss a dose of fosamprenavir calcium tablets, to take it as soon as they remember. Advise patients not to double their next dose or take more than the prescribed dose [see Dosage and Administration (2)].
-
Patient Information
Fosamprenavir Calcium Tablets
(fos″ am pren′ a vir kal′ see um)
What is the most important information I should know about fosamprenavir calcium tablets?
Fosamprenavir calcium tablets can interact with other medicines and cause serious side effects. It is important to know the medicines that should not be taken with fosamprenavir calcium tablets. See the section “Who should not take fosamprenavir calcium tablets?” Fosamprenavir calcium tablets can cause serious side effects, including:
- Severe skin reactions. Fosamprenavir calcium tablets may cause severe or life-threatening skin reactions or rash.
If you get a rash with any of the following symptoms, stop taking fosamprenavir calcium tablets and call your healthcare provider or get medical help right away:
-
- o hives or sores in your mouth, or your skin blisters and peels
- o trouble swallowing or breathing
- o swelling of your face, eyes, lips, tongue, or throat
For more information about side effects, see “What are the possible side effects of fosamprenavir calcium tablets?”
What are fosamprenavir calcium tablets?
Fosamprenavir calcium tablets are a prescription medicine that is used together with other antiretroviral medicines to treat human immunodeficiency virus 1 (HIV-1) infection.
HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS).
It is not known if fosamprenavir is safe and effective in children younger than 4 weeks of age.
Do not take fosamprenavir calcium tablets if you:
- are allergic to amprenavir, fosamprenavir calcium, or any of the ingredients in fosamprenavir calcium tablets. See the end of this leaflet for a complete list of ingredients in fosamprenavir calcium tablets.
-
take any of the following medicines:
- o alfuzosin
- o rifampin
- o
ergot including:
- ▪ dihydroergotamine mesylate
- ▪ ergonovine
- ▪ ergotamine tartrate
- ▪ methylergonovine
- o cisapride
- o St. John’s wort (Hypericum perforatum)
- o lomitapide
- o lovastatin
- o simvastatin
- o pimozide
- o delavirdine mesylate
- o sildenafil (REVATIO), for treatment of pulmonary arterial hypertension
- o triazolam
- o midazolam, when taken by mouth
Serious problems can happen if you or your child take any of the medicines listed above with fosamprenavir calcium tablets.
If you are taking fosamprenavir calcium tablets with ritonavir, do not take the following medicines:
-
- o flecainide
- o propafenone
- o lurasidone
Before taking fosamprenavir calcium tablets, tell your healthcare provider about all of your medical conditions, including if you:
- are allergic to medicines that contain sulfa.
- have or have had liver problems, including hepatitis B or C virus infection.
- have kidney problems.
- have high blood sugar (diabetes).
- have hemophilia.
- have high cholesterol.
-
are pregnant or plan to become pregnant. It is not known if fosamprenavir calcium tablets will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant during treatment with fosamprenavir calcium tablets.
- o Fosamprenavir calcium tablets may reduce how well hormonal contraceptives (birth control pills) work. Females who may become pregnant should use a different form of birth control or an additional barrier method of birth control during treatment with fosamprenavir calcium tablets.
Pregnancy Registry. There is a pregnancy registry for women who take fosamprenavir calcium tablets during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
-
are breastfeeding or plan to breastfeed. Do not breastfeed if you take fosamprenavir calcium tablets.
- o You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- o It is not known if fosamprenavir can pass to your baby in your breast milk.
- o Talk with your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines interact with fosamprenavir calcium tablets. Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with fosamprenavir calcium tablets.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take fosamprenavir calcium tablets with other medicines.
How should I take fosamprenavir calcium tablets?
- Take fosamprenavir calcium tablets exactly as your healthcare provider tells you to take them.
- If you miss a dose of fosamprenavir calcium tablets, take it as soon as you remember. Do not take 2 doses at the same time or take more than your healthcare provider tells you to take.
- Stay under the care of a healthcare provider during treatment with fosamprenavir calcium tablets.
- If your child is taking fosamprenavir calcium tablets, your child’s healthcare provider will decide the right dose based on your child’s weight.
- Fosamprenavir calcium tablets may be taken with or without food.
- Do not run out of fosamprenavir calcium tablets. The virus in your blood may increase and the virus may become harder to treat. When your supply starts to run low, get more from your healthcare provider or pharmacy.
- If you take too many fosamprenavir calcium tablets, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of fosamprenavir calcium tablets?
Fosamprenavir calcium tablets may cause serious side effects including:
- See “What is the most important information I should know about fosamprenavir calcium tablets?”
- Liver problems. Your healthcare provider should do blood tests before and during your treatment with fosamprenavir calcium tablets to check your liver function. Some people with liver problems, including hepatitis B or C, may have an increased risk of developing worsening liver problems during treatment with fosamprenavir calcium tablets.
- Diabetes and high blood sugar (hyperglycemia). Some people who take protease inhibitors, including fosamprenavir calcium tablets, can get high blood sugar, develop diabetes, or your diabetes can get worse. Tell your healthcare provider if you notice an increase in thirst or urinate often during treatment with fosamprenavir calcium tablets.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Call your healthcare provider right away if you start having new symptoms after starting your HIV-1 medicine.
- Increase in body fat. An increase in body fat can happen in people who take protease inhibitors, including fosamprenavir calcium tablets. The exact cause and long-term health effects of these conditions are not known.
- Changes in blood tests. Some people have changes in blood tests while taking fosamprenavir calcium tablets. These include an increase in liver function tests, blood fat levels (cholesterol and triglycerides) and decrease in red blood cells. Your healthcare provider should do regular blood tests before and during your treatment with fosamprenavir calcium tablets.
- Increased bleeding problems in some people with hemophilia. Some people with hemophilia have increased bleeding with protease inhibitors, including fosamprenavir calcium tablets.
-
Kidney stones. Some people have developed kidney stones during treatment with fosamprenavir calcium tablets. Tell your healthcare provider right away if you develop any of the following signs or symptoms of kidney stones:
- o pain in your side
- o blood in your urine
- o pain when you urinate
The most common side effects of fosamprenavir calcium tablets in adults include:
- nausea
- vomiting
- rash
- diarrhea
- headache
The most common side effects of fosamprenavir in children include vomiting and decrease in white blood cells.
These are not all the possible side effects of fosamprenavir calcium tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store fosamprenavir calcium tablets?
- Store fosamprenavir calcium tablets at room temperature between 20° to 25°C (68° to 77°F).
- Keep the bottle of fosamprenavir calcium tablets tightly closed.
- Fosamprenavir calcium tablets come in a child-resistant package.
Keep fosamprenavir calcium tablets and all medicines out of the reach of children.
General information about the safe and effective use of fosamprenavir calcium tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use fosamprenavir calcium tablets for a condition for which they were not prescribed. Do not give fosamprenavir calcium tablets to other people, even if they have the same symptoms you have. They may harm them. You can ask your pharmacist or healthcare provider for information about fosamprenavir calcium tablets that is written for health professionals.
What are the ingredients in fosamprenavir calcium tablets?
Active ingredient: fosamprenavir calcium
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, red iron oxide, silicified microcrystalline cellulose, titanium dioxide and triacetin.
Manufactured for: Mylan Pharmaceuticals Inc., Morgantown, WV 26505 U.S.A.
Manufactured by: Mylan Laboratories Limited,Hyderabad — 500 096, India
The brand names listed are trademarks of their respective owners.
For more information, call Mylan at 1-877-446-3679 (1-877-4-INFO-RX).
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Manufactured by:
Mylan Laboratories Limited
Hyderabad — 500 096, India75068087
Revised: 7/2019
MX:FOSM:R6 -
PRINCIPAL DISPLAY PANEL – 700 mg
NDC: 0378-3520-91
Fosamprenavir
Calcium
Tablets
700 mgALERT: Find out about medicines that should NOT
be taken with Fosamprenavir Calcium Tablets.Note to Pharmacist:
Do not cover ALERT box
with pharmacy label.Rx only 60 Tablets
Each film-coated tablet contains
700 mg of fosamprenavir as
fosamprenavir calcium.Usual Dosage: See accompanying
prescribing information.For use in combination regimens with
or without ritonavir.Keep this and all medication out of the
reach of children.Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Made in India
Mylan.com
RMX3520D2
Dispense in original container.
Keep container tightly closed.
Code No.: MH/DRUGS/25/NKD/89
-
INGREDIENTS AND APPEARANCE
FOSAMPRENAVIR CALCIUM
fosamprenavir calcium tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0378-3520 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOSAMPRENAVIR CALCIUM (UNII: ID1GU2627N) (AMPRENAVIR - UNII:5S0W860XNR) FOSAMPRENAVIR 700 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color PINK Score no score Shape OVAL (modified capsule shaped) Size 21mm Flavor Imprint Code M;FT7 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0378-3520-91 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/18/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204060 09/18/2017 Labeler - Mylan Pharmaceuticals Inc. (059295980)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.