KETOROLAC TROMETHAMINE solution
Ketorolac Tromethamine by
Drug Labeling and Warnings
Ketorolac Tromethamine by is a Prescription medication manufactured, distributed, or labeled by Akorn Inc., Akorn Operating Company LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KETOROLAC TROMETHAMINE OPHTHALMIC SOLUTION, 0.4% safely and effectively. See full prescribing information for KETOROLAC TROMETHAMINE OPHTHALMIC SOLUTION, 0.4%.
KETOROLAC TROMETHAMINE ophthalmic solution, 0.4%, for topical ophthalmic use
Initial U.S. Approval: 1991INDICATIONS AND USAGE
Ketorolac tromethamine ophthalmic solution is a nonsteroidal, anti-inflammatory drug (NSAID) indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery. (1)
DOSAGE AND ADMINISTRATION
One drop of ketorolac tromethamine ophthalmic solution should be applied in the operated eye 4 times per day as needed for pain and burning/stinging for up to 4 days following corneal refractive surgery. (2.1)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing 4 mg/mL ketorolac tromethamine in a 10 mL size bottle filled with 5 mL of solution. (3)
CONTRAINDICATIONS
Hypersensitivity to any component of this product. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions occurring in 1% to 5% of patients included conjunctival hyperemia, corneal infiltrates, headache, ocular edema and ocular pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Akorn, Inc. at 1-800-932-5676 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Use With Other Topical Ophthalmic Medications
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Delayed Healing
5.2 Cross-Sensitivity or Hypersensitivity
5.3 Increased Bleeding Time
5.4 Corneal Effects
5.5 Contact Lens Wear
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
The recommended dose of ketorolac tromethamine ophthalmic solution is one drop four times a day in the operated eye as needed for pain and burning/stinging for up to 4 days following corneal refractive surgery.
2.2 Use With Other Topical Ophthalmic Medications
Ketorolac tromethamine ophthalmic solution has been safely administered in conjunction with other topical ophthalmic medications such as alpha-agonists, antibiotics, beta-blockers, carbonic anhydrase inhibitors, cycloplegics, and mydriatics. Drops should be administered at least 5 minutes apart.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Delayed Healing
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
5.2 Cross-Sensitivity or Hypersensitivity
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other NSAIDs. There have been reports of bronchospasm or exacerbation of asthma associated with the use of ketorolac tromethamine ophthalmic solution in patients who have either a known hypersensitivity to aspirin/non-steroidal anti-inflammatory drugs or a past medical history of asthma. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
5.3 Increased Bleeding Time
With some NSAIDs, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied nonsteroidal anti-inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
It is recommended that ketorolac tromethamine ophthalmic solution be used with caution in patients with known bleeding tendencies or who are receiving other medications, which may prolong bleeding time.
5.4 Corneal Effects
Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration, or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events which may become sight threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 1 day prior to surgery or use beyond 14 days post-surgery may increase patient risk for the occurrence and severity of corneal adverse events.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
Delayed Healing [see Warnings and Precautions (5.1)]
- Cross-Sensitivity or Hypersensitivity [see Warnings and Precautions (5.2)]
- Increased Bleeding Time [see Warnings and Precautions (5.3)]
- Corneal Effects [see Warnings and Precautions (5.4)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The most frequently reported adverse reactions for ketorolac tromethamine ophthalmic solution occurring in approximately 1% to 5% of the overall study population were conjunctival hyperemia, corneal infiltrates, headache, ocular edema, and ocular pain.
The most frequent adverse reactions reported with the use of ketorolac tromethamine ophthalmic solutions have been transient stinging and burning on instillation. These reactions were reported by up to 40% of patients participating in clinical trials.
Other adverse reactions occurring approximately in 1% to 10% of the time during treatment with other ketorolac tromethamine ophthalmic solutions included allergic reactions, corneal edema, iritis, ocular inflammation, ocular irritation, superficial keratitis, and superficial ocular infections.
Other adverse reactions reported rarely with the use of ketorolac tromethamine ophthalmic solutions included: corneal ulcer, eye dryness, and visual disturbance (blurry vision).
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of ketorolac tromethamine ophthalmic solutions in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to topical ketorolac tromethamine ophthalmic solutions or a combination of these factors, include bronchospasm or exacerbation of asthma, corneal erosion, corneal perforation, corneal thinning and corneal melt, and epithelial breakdown [see Warnings and Precautions (5.2, 5.4)].
- 8 USE IN SPECIFIC POPULATIONS
-
11 DESCRIPTION
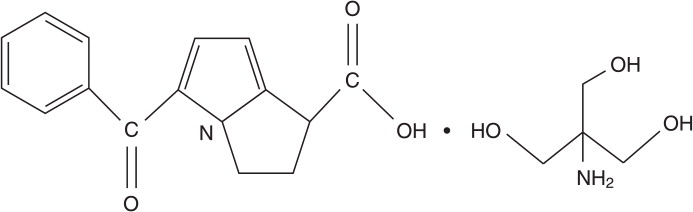
Ketorolac tromethamine ophthalmic solution, 0.4% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for ophthalmic use. Its chemical name is (±)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1), and its molecular weight is 376.40. Its molecular formula is C19H24N2O6 and it has the following structure:
Ketorolac tromethamine ophthalmic solution is supplied as a sterile isotonic aqueous 0.4% solution, with a pH of approximately 7.4. Ketorolac tromethamine ophthalmic solution contains a racemic mixture of R(+) and S-(-)-ketorolac tromethamine. Ketorolac tromethamine may exist in three crystal forms. All forms are equally soluble in water. The pKa of ketorolac is 3.5. This white to off-white crystalline substance discolors on prolonged exposure to light. The osmolality of ketorolac tromethamine ophthalmic solution is 290 mOsmol/kg.
Each mL of ketorolac tromethamine ophthalmic solution, 0.4% contains: Active: ketorolac tromethamine 0.4%. Inactives: edetate disodium 0.015%; octoxynol 40; purified water; sodium chloride; and hydrochloric acid and/or sodium hydroxide to adjust pH. Preservative: benzalkonium chloride 0.006%.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug which, when administered systemically, has demonstrated analgesic, anti-inflammatory, and anti-pyretic activity. The mechanism of its action is thought to be due to its ability to inhibit prostaglandin biosynthesis.
12.3 Pharmacokinetics
One drop of 0.5% ketorolac tromethamine ophthalmic solution was instilled into one eye and one drop of vehicle into the other eye TID in 26 healthy subjects. Five of 26 subjects had detectable concentrations of ketorolac in their plasma (range 11 to 23 ng/mL) at day 10 during topical ocular treatment.
Two drops of 0.5% ketorolac tromethamine ophthalmic solution instilled into the eyes of patients 12 hours and 1 hour prior to cataract extraction achieved a mean ketorolac concentration of 95 ng/mL in the aqueous humor of 8 of 9 eyes tested (range 40 to 170 ng/mL).
-
14 CLINICAL STUDIES
In two double-masked, multi-centered, parallel-group studies, 313 patients who had undergone photorefractive keratectomy received ketorolac tromethamine ophthalmic solution or its vehicle QID for up to 4 days. Significant differences favored ketorolac tromethamine ophthalmic solution for the reduction of ocular pain and burning/stinging following photorefractive keratectomy surgery.
Results from clinical studies indicate that ketorolac tromethamine has no significant effect upon intraocular pressure.
The safety and effectiveness of ketorolac tromethamine ophthalmic solution in post-cataract surgery patients has not been established.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Ketorolac tromethamine ophthalmic solution, 0.4% is supplied sterile, in a white LDPE boston round bottle with a LDPE natural-colored dropper tip and a gray short skirt cap as follows:
5 mL in 10 mL bottle - NDC: 17478-208-10
-
17 PATIENT COUNSELING INFORMATION
Slow or Delayed Healing
Inform patients of the possibility that slow or delayed healing may occur while using nonsteroidal anti-inflammatory drugs (NSAIDs).
Avoiding Contamination of the Product
Instruct patients to avoid allowing the tip of the bottle to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions. Also, to avoid the potential for cross-contamination, the patient should be advised to use one bottle for each eye following bilateral ocular surgery. The use of the same bottle of topical eye drops for both eyes following bilateral ocular surgery is not recommended.
Contact Lens Wear
Advise patients that ketorolac tromethamine ophthalmic solution should not be administered while wearing contact lenses.
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 17478-208-10
Ketorolac
Tromethamine
Ophthalmic Solution
0.4%
Rx only 5 mL
Akorn Logo
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 17478-208-10
Ketorolac
Tromethamine
Ophthalmic
Solution
0.4%
FOR TOPICAL OPHTHALMIC
USE ONLY
5 mL
Rx only Akorn Logo
-
INGREDIENTS AND APPEARANCE
KETOROLAC TROMETHAMINE
ketorolac tromethamine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17478-208 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ketorolac tromethamine (UNII: 4EVE5946BQ) (ketorolac - UNII:YZI5105V0L) ketorolac tromethamine 4 mg in 1 mL Inactive Ingredients Ingredient Name Strength benzalkonium chloride (UNII: F5UM2KM3W7) edetate disodium (UNII: 7FLD91C86K) octoxynol-40 (UNII: 9T1C662FKS) sodium chloride (UNII: 451W47IQ8X) hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-208-10 1 in 1 CARTON 11/05/2009 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078399 11/05/2009 Labeler - Akorn Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn Inc. 603980319 MANUFACTURE(17478-208) , ANALYSIS(17478-208) , REPACK(17478-208)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.