DERCUT- euphorbia resinifera resin, goldenseal, wood creosote, toxicodendron pubescens shoot, sempervivum tectorum leaf, bellis perennis, vinca minor, and viola tricolor ointment
DERCUT by
Drug Labeling and Warnings
DERCUT by is a Homeopathic medication manufactured, distributed, or labeled by PEKANA Naturheilmittel GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses1
- 1 Application of this homeopathic remedy for the designated usage is exclusively based on homeopathic experience. These "Uses" have not been evaluated by the Food and Drug Administration. With severe forms of this disease, a clinically proven therapy is indicated.
-
Warnings
For external use only. Do not use on open wounds!
If symptoms persist, contact a licensed practitioner.
If you have known sensitivity to any of the ingredients, please consult your licensed practitioner before use.
If you are pregnant or nursing a baby, seek the advice of a health care professional before use.
- Directions
- Other information
- Tamper Evident
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
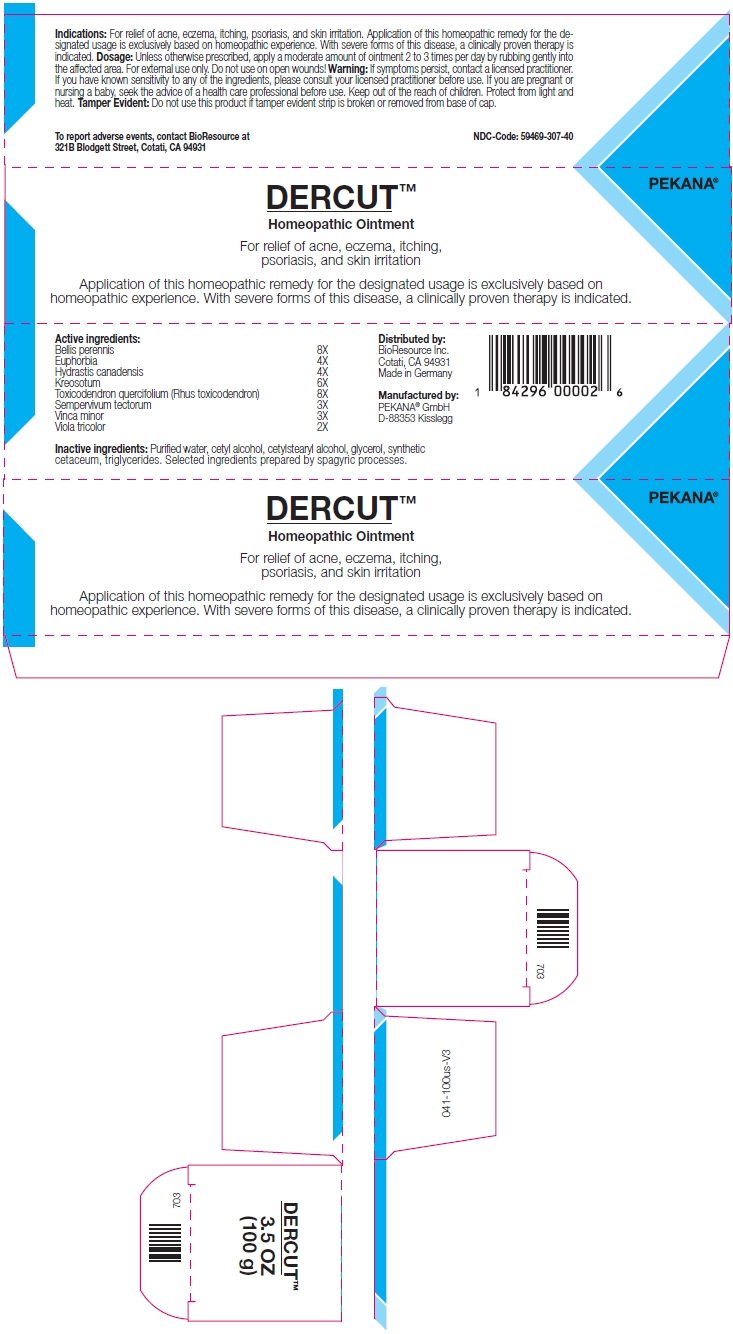
- PRINCIPAL DISPLAY PANEL - 100 g Tube Box
-
INGREDIENTS AND APPEARANCE
DERCUT
euphorbia resinifera resin, goldenseal, wood creosote, toxicodendron pubescens shoot, sempervivum tectorum leaf, bellis perennis, vinca minor, and viola tricolor ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59469-307 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUPHORBIA RESINIFERA RESIN (UNII: 1TI1O9028K) (Euphorbia resinifera Resin - UNII:1TI1O9028K) EUPHORBIA RESINIFERA RESIN 4 [hp_X] in 100 g GOLDENSEAL (UNII: ZW3Z11D0JV) (Goldenseal - UNII:ZW3Z11D0JV) GOLDENSEAL 4 [hp_X] in 100 g WOOD CREOSOTE (UNII: 3JYG22FD73) (WOOD CREOSOTE - UNII:3JYG22FD73) WOOD CREOSOTE 6 [hp_X] in 100 g TOXICODENDRON PUBESCENS SHOOT (UNII: 46PYZ1F82M) (TOXICODENDRON PUBESCENS SHOOT - UNII:46PYZ1F82M) TOXICODENDRON PUBESCENS SHOOT 8 [hp_X] in 100 g Sempervivum tectorum Leaf (UNII: 3DGJ7BUA01) (Sempervivum tectorum Leaf - UNII:3DGJ7BUA01) Sempervivum tectorum Leaf 3 [hp_X] in 100 g BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 8 [hp_X] in 100 g VINCA MINOR (UNII: WGM46PQF02) (VINCA MINOR - UNII:WGM46PQF02) VINCA MINOR 3 [hp_X] in 100 g VIOLA TRICOLOR (UNII: 9Q24RAI43V) (VIOLA TRICOLOR - UNII:9Q24RAI43V) VIOLA TRICOLOR 2 [hp_X] in 100 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Cetyl Alcohol (UNII: 936JST6JCN) SPERMACETI (UNII: P86KHA8V3K) Glycerin (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Alcohol (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59469-307-40 1 in 1 BOX 06/24/2019 1 100 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 59469-307-30 1 in 1 BOX 06/24/2019 2 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 06/24/2019 Labeler - PEKANA Naturheilmittel GmbH (320344542) Establishment Name Address ID/FEI Business Operations PEKANA Naturheilmittel GmbH 320344542 MANUFACTURE(59469-307)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.