XYNTHA (antihemophilic factor- recombinant kit
Xyntha by
Drug Labeling and Warnings
Xyntha by is a Other medication manufactured, distributed, or labeled by Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC, Wyeth Farma SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XYNTHA safely and effectively. See full prescribing information for XYNTHA.
XYNTHA® (antihemophilic factor [recombinant]) lyophilized powder for solution, for intravenous injection
Initial U.S. Approval: 2008INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only (2)
- The required dose is determined using the following formula:
Required units = body weight (kg) × desired factor VIII rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL), where IU = International Unit. (2.1) - Frequency of XYNTHA administration is determined by the type of bleeding episode and the recommendation of the treating physician. (2.1, 2.2)
DOSAGE FORMS AND STRENGTHS
XYNTHA is available as lyophilized powder in single-use vials containing nominally 250, 500, 1000, or 2000 IU. (3)
CONTRAINDICATIONS
Do not use in patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product or its components, including hamster proteins. (4)
WARNINGS AND PRECAUTIONS
- Anaphylaxis and severe hypersensitivity reactions are possible. Patients may develop hypersensitivity to hamster protein, which is present in trace amounts in XYNTHA. Should such reactions occur, discontinue treatment with the product and administer appropriate treatment. (5.1)
- Development of neutralizing antibodies has been reported in patients using XYNTHA. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures factor VIII inhibitor concentration. (5.2, 5.3, 6.2)
ADVERSE REACTIONS
- The most common adverse reactions (≥10%) with XYNTHA in adult and pediatric previously treated patients (PTPs) were headache, arthralgia, pyrexia, and cough. (6)
- Across all studies, 4 subjects developed factor VIII inhibitors (2.4%). (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Wyeth Pharmaceuticals LLC. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
Pediatrics: Half-lives are shorter, volumes of distribution are larger, and recovery is lower after XYNTHA administration in children. Higher or more frequent dosing may be needed. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2019
- The required dose is determined using the following formula:
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation and Reconstitution
2.3 Administration
2.4 Use of a XYNTHA Vial Kit with a XYNTHA SOLOFUSE Kit
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Neutralizing Antibodies
5.3 Monitoring Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
XYNTHA, Antihemophilic Factor (Recombinant), is indicated for use in adults and children with hemophilia A (congenital factor VIII deficiency) for:
- On-demand treatment and control of bleeding episodes
- Perioperative management
XYNTHA does not contain von Willebrand factor, and therefore is not indicated in patients with von Willebrand's disease.
-
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
2.1 Dose
- Dosage and duration of treatment depend on the severity of the factor VIII deficiency, the location and extent of bleeding, and the patient's clinical condition. Titrate the administered doses to the patient's clinical response.
- One International Unit (IU) of factor VIII activity corresponds approximately to the quantity of factor VIII in one milliliter of normal human plasma. The calculation of the required dosage of factor VIII is based upon the empirical finding that, on average, 1 IU of factor VIII per kg body weight raises the plasma factor VIII activity by approximately 2 IU/dL.2
The expected in vivo peak increase in factor VIII level expressed as IU/dL (or % normal) can be estimated using the following formulas:
Dosage (International Units) = body weight (kg) × desired factor VIII rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL)
or
IU/dL (or % normal) = Total Dose (IU)/body weight (kg) × 2 [IU/dL]/[IU/kg]
On-demand treatment and Control of Bleeding Episodes
A guide for dosing XYNTHA for on-demand treatment and control of bleeding episodes is provided in Table 1. Maintain the plasma factor VIII activity at or above the levels (in % of normal or in IU/dL) outlined in Table 1 for the indicated period.
Table 1: Dosing for On-demand treatment and Control of Bleeding Episodes Type of Bleeding Episode Factor VIII Level Required (IU/dL or % of normal) Frequency of Doses
(hours)Duration of Therapy Minor Early hemarthrosis, minor muscle or oral bleeds. 20–40 12–24 At least 1 day, depending upon the severity of the bleeding episode. Moderate Bleeding into muscles.
Mild head trauma.
Bleeding into the oral cavity.30–60 12–24 3–4 days or until adequate local hemostasis is achieved. Major Gastrointestinal bleeding.
Intracranial, intra-abdominal, or intrathoracic bleeding.
Fractures.60–100 8–24 Until bleeding is resolved. Perioperative Management
A guide for dosing XYNTHA during surgery (perioperative management) is provided in Table 2. Maintain the plasma factor VIII activity level at or above the level (in % of normal or in IU/dL) outlined in Table 2 for the indicated period. Monitor the replacement therapy by means of plasma factor VIII activity.
Table 2: Dosing for Perioperative Management Type of Surgery Factor VIII Level Required (IU/dL or % of normal) Frequency of Doses
(hours)Duration of Therapy
(days)Minor Minor operations, including tooth extraction. 30–60 12–24 3–4 days or until adequate local hemostasis is achieved. For tooth extraction, a single infusion plus oral antifibrinolytic therapy within 1 hour may be sufficient. Major Major operations. 60–100 8–24 Until threat is resolved, or in the case of surgery, until adequate local hemostasis and wound healing are achieved. 2.2 Preparation and Reconstitution
Preparation
- Always wash hands before performing the following procedures.
- Use aseptic technique during the reconstitution procedures.
- Use all components in the reconstitution and administration of this product as soon as possible after opening their sterile containers to minimize unnecessary exposure to the atmosphere.
Note:
- If the patient uses more than one vial of XYNTHA per infusion, reconstitute each vial according to the following instructions. Remove the diluent syringe, leaving the vial adapter in place. Use a separate 10 milliliter or larger luer lock syringe (not included in this kit) to draw back the reconstituted contents of each vial. Do not detach the diluent syringe or the large luer lock syringe until ready to attach the large luer lock syringe to the next vial adapter.
- If the patient uses one vial of XYNTHA with one XYNTHA SOLOFUSE for the infusion, reconstitute the vial and the syringe according to the instructions for each respective product kit. Use a separate 10 milliliter or larger luer lock syringe (not included in this kit) to draw back the reconstituted contents of the vial and the syringe. [see Dosage and Administration (2.4)]
Reconstitution
- Allow the XYNTHA vial and the prefilled diluent syringe to reach room temperature.
- Remove the plastic flip-top cap from the XYNTHA vial to expose the central portions of the rubber stopper.
- Wipe the top of the vial with the alcohol swab provided, or use another antiseptic solution, and allow to dry. After cleaning, do not touch the rubber stopper or allow it to touch any surface.
- Peel back the cover from the clear plastic vial adapter package. Do not remove the adapter from the package.
- Place the XYNTHA vial on a flat surface. While holding the adapter package, place the vial adapter over the XYNTHA vial and press down firmly on the package until the adapter spike penetrates the vial stopper.
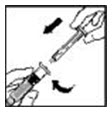
- Grasp the plunger rod as shown in the diagram. Avoid contact with the shaft of the plunger rod. Attach the threaded end of the plunger rod to the diluent syringe plunger by pushing and turning firmly.
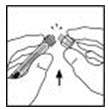
- Break off the tamper-resistant plastic tip cap from the diluent syringe by snapping the perforation of the cap. Do not touch the inside of the cap or the syringe tip. The diluent syringe may need to be recapped (if not administering reconstituted XYNTHA immediately), so place the cap on its top on a clean surface in a spot where it would be least likely to become environmentally contaminated.
- Lift the package away from the adapter and discard the package.
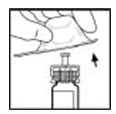
- Place the XYNTHA vial, with the adapter attached, on a flat surface. Connect the diluent syringe to the vial adapter by inserting the tip into the adapter opening while firmly pushing and turning the syringe clockwise until secured.
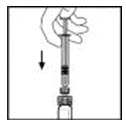
- Slowly depress the plunger rod to inject all the diluent into the XYNTHA vial.
- Without removing the syringe, gently swirl the contents of the XYNTHA vial until the powder is dissolved.
Note: The final solution should be inspected visually for particulate matter before administration. The solution should be clear to slightly opalescent and colorless. If it is not, discard the solution and use a new kit. - Invert the XYNTHA vial and slowly draw the solution into the syringe.
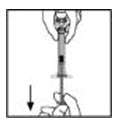
- Detach the syringe from the vial adapter by gently pulling and turning the syringe counterclockwise. Discard the empty XYNTHA vial with the adapter attached.
Note:
- If the solution is not used immediately, carefully replace the syringe cap. Do not touch the syringe tip or the inside of the cap.
- Store the reconstituted solution at room temperature prior to administration, but use within 3 hours after reconstitution.
- XYNTHA, when reconstituted, contains polysorbate 80, which is known to increase the rate of di-(2-ethylhexyl) phthalate (DEHP) extraction from polyvinyl chloride (PVC). This should be considered during the preparation and administration of XYNTHA, including storage time elapsed in a PVC container following reconstitution. The tubing of the infusion set included with this kit does not contain DEHP.
2.3 Administration
For intravenous infusion after reconstitution only.
Inspect the final XYNTHA solution visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be clear to slightly opalescent and colorless. If it is not, discard the solution and use a new kit.
Use the tubing and the prefilled diluent syringe provided in this kit or a single sterile disposable plastic syringe. Do not administer XYNTHA in the same tubing or container with other medicinal products.
- Attach the syringe to the luer end of the infusion set tubing provided.
- Apply a tourniquet and prepare the injection site by wiping the skin well with an alcohol swab provided in the kit.
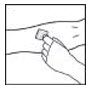
- Remove the protective needle cover and perform venipuncture. Insert the needle on the infusion set tubing into the vein, and remove the tourniquet. Verify proper needle placement.
- Inject the reconstituted XYNTHA product intravenously over several minutes. The rate of administration should be determined by the patient's comfort level.
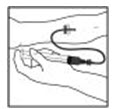
- After infusing XYNTHA, remove and discard the infusion set. The amount of drug product left in the infusion set will not affect treatment.
Note: Dispose of all unused solution, the empty vial(s), and other used medical supplies in an appropriate container.
2.4 Use of a XYNTHA Vial Kit with a XYNTHA SOLOFUSE Kit
These instructions are for the use of only one XYNTHA Vial Kit with one XYNTHA SOLOFUSE Kit. For further information, please contact the Medical Information Department at Wyeth Pharmaceuticals, 1-800-438-1985.
- Reconstitute the XYNTHA vial using the instructions described in Preparation and Reconstitution [see Dosage and Administration (2.2)].
- Detach the empty diluent syringe from the vial adapter by gently turning and pulling the syringe counterclockwise, leaving the contents in the XYNTHA vial with the vial adapter in place.
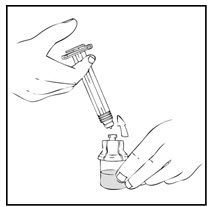
- Reconstitute the XYNTHA SOLOFUSE using the instructions included with the product kit, remembering to remove most, but not all, of the air from the drug product chamber.
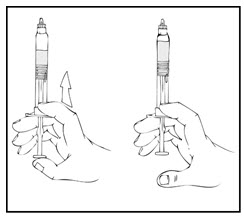
- After removing the protective blue vented cap, connect the XYNTHA SOLOFUSE to the vial adapter by inserting the tip into the adapter opening while firmly pushing and turning the syringe clockwise until secured.
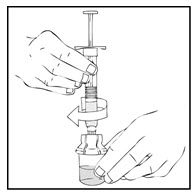
- Slowly depress the plunger rod of the XYNTHA SOLOFUSE until the contents empty into the XYNTHA vial. The plunger rod may move back slightly after release.
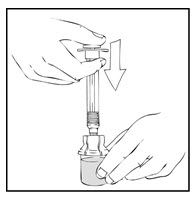
- Detach and discard the empty XYNTHA SOLOFUSE from the vial adapter.
Note: If the syringe turns without detaching from the vial adapter, grasp the white collar and turn.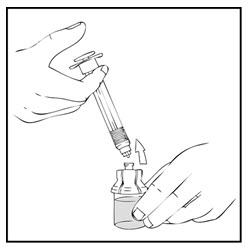
- Connect a sterile 10 milliliter or larger luer lock syringe to the vial adapter. Inject some air into the vial to make withdrawing the vial contents easier.
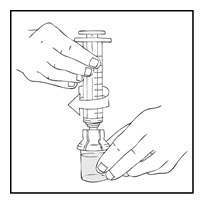
- Invert the vial and slowly draw the solution into the large luer lock syringe.
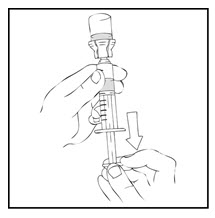
- Detach the syringe from the vial adapter by gently turning and pulling the syringe counterclockwise. Discard the vial with the adapter attached.
- Attach the infusion set to the large luer lock syringe as directed [see Dosage and Administration (2.3)].
-
3 DOSAGE FORMS AND STRENGTHS
XYNTHA is available as a white to off-white lyophilized powder in the following nominal dosages:
- 250 International Units
- 500 International Units
- 1000 International Units
- 2000 International Units
Each XYNTHA vial has the actual recombinant factor VIII (rFVIII) potency in International Units stated on the label.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Allergic-type hypersensitivity reactions, including anaphylaxis, are possible with XYNTHA. Inform patients of the early signs or symptoms of hypersensitivity reactions (including hives [rash with itching], generalized urticaria, chest tightness, wheezing, and hypotension) and anaphylaxis. Discontinue XYNTHA if hypersensitivity symptoms occur and administer appropriate emergency treatment.
XYNTHA contains trace amounts of hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
5.2 Neutralizing Antibodies
Inhibitors have been reported following administration of XYNTHA. Monitor patients for the development of factor VIII inhibitors by appropriate clinical observations and laboratory tests. If expected factor VIII activity plasma levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures factor VIII inhibitor concentration to determine if a factor VIII inhibitor is present [see Warnings and Precautions (5.3)].4,5,6,7,8,9,10,11,12
5.3 Monitoring Laboratory Tests
- Use individual factor VIII values for recovery and, if clinically indicated, other pharmacokinetic characteristics to guide dosing and administration.
- Monitor plasma factor VIII activity levels by the one-stage clotting assay to confirm that adequate factor VIII levels have been achieved and are maintained, when clinically indicated [see Dosage and Administration (2)].
- Monitor for development of factor VIII inhibitors. Perform assay to determine if factor VIII inhibitor is present when expected factor VIII activity plasma levels are not attained, or when bleeding is not controlled with the expected dose of XYNTHA. Use Bethesda Units (BU) to titer inhibitors.
-
6 ADVERSE REACTIONS
The most common adverse reactions (≥10%) with XYNTHA in adult and pediatric previously treated patients (PTPs) were headache, arthralgia, pyrexia, and cough.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
XYNTHA was evaluated in five completed clinical studies (N=178), comprising four studies with adult and pediatric PTPs.
The safety and efficacy of XYNTHA was evaluated in two completed pivotal studies. In the first study (n=94), safety and efficacy were examined in PTPs with severe to moderately severe hemophilia A (factor VIII activity in plasma [FVIII:C] ≤2%) who received XYNTHA for routine prophylaxis and on-demand treatment. Ninety-four subjects received at least one dose of XYNTHA, resulting in a total of 6,775 infusions [see Clinical Studies (14)]. The second study (n=30) examined the use of XYNTHA for surgical prophylaxis in PTPs with severe to moderately severe hemophilia A (FVIII:C ≤2%) who required elective major surgery and were expected to receive XYNTHA replacement therapy for at least 6 days post-surgery. All subjects received at least one dose of XYNTHA, resulting in 1,161 infusions. One subject received XYNTHA for a pre-surgery pharmacokinetic assessment only and did not undergo surgery [see Clinical Studies (14)].
Across all studies, safety was evaluated in 72 pediatric PTPs <17 years of age (46 subjects <6 years of age (4 subjects were 0 to <2 years of age), 4 subjects 6 to <12 years of age, and 22 adolescents, 12 to <17 years of age). A total of 13,109 infusions of XYNTHA were administered with a median dose per infusion of 28 IU/kg (min-max: 6–108 IU/kg).
Across all studies, the most common adverse reactions (≥10%) with XYNTHA in adult and pediatric PTPs were headache (24%), arthralgia (23%), pyrexia (23%), and cough (12%). Other adverse reactions reported in ≥5% of subjects were: diarrhea (8%), vomiting (8%), and asthenia (6%).
6.2 Immunogenicity
There is a potential for immunogenicity with therapeutic proteins. The development of factor VIII inhibitors with XYNTHA was evaluated in 167 adult and pediatric PTPs with at least 50 exposure days (EDs). Laboratory-based assessments for FVIII inhibitor (partial Nijmegen modification of the Bethesda inhibitor assay) were conducted in the clinical studies. The criterion for a positive FVIII result test result was ≥0.6 BU/mL. Across all studies, 4 subjects developed factor VIII inhibitors (2.4%).
The completed clinical studies for XYNTHA examined 178 subjects (30 for surgical prophylaxis) who had previously been treated with factor VIII (PTPs). In the first safety and efficacy study, factor VIII inhibitors were detected in two of 89 subjects (2.2%) who completed ≥50 EDs. In a Bayesian statistical analysis, results from this study were used to update PTP results from a prior supporting study using XYNTHA manufactured at the initial facility (with one de novo and two recurrent inhibitors observed in 110 subjects) and the experience with predecessor product (with one inhibitor observed in 113 subjects). The Bayesian analysis indicated that the population inhibitor rate for XYNTHA, an estimate of the 95% upper limit of the true inhibitor rate, was 4.17%.
None of the PTPs developed anti-CHO (Chinese hamster ovary) or anti-TN8.2 antibodies. One PTP developed anti-FVIII antibodies; but, this subject did not develop an inhibitor.
In the surgery study, one low titer persistent inhibitor and one transient false-positive inhibitor were reported. In this study, one surgical subject developed anti-CHO cell antibodies with no associated allergic reaction. One subject developed anti-FVIII antibodies; but, this subject did not develop an inhibitor.
Across all studies, immunogenicity was evaluated in 64 pediatric PTPs <17 years of age with at least 50 EDs (43 children <6 years of age, 4 subjects <12 years of age, and 17 adolescents, 12 to <17 years of age). Of these, 2 pediatric subjects developed an inhibitor.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody, including neutralizing antibody, positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparisons of the incidence of antibodies to XYNTHA with the incidence of antibodies to other products may be misleading.
6.3 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following postmarketing adverse reaction has been reported for XYNTHA:
Inadequate therapeutic response
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
It is not known whether XYNTHA can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Animal reproduction studies have not been conducted with XYNTHA.
There is no information available on the effect of factor VIII replacement therapy on labor and delivery. XYNTHA should be used only if clinically indicated.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of XYNTHA in human milk, the effect on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for XYNTHA and any potential adverse effects on the breastfed child from XYNTHA or from the underlying maternal condition.
8.4 Pediatric Use
In the first completed open-label safety and efficacy study of XYNTHA (n=94), of the 18 adolescent subjects 12 to <17 years of age with severe to moderately severe hemophilia A (FVIII:C ≤2%), who were previously treated with at least 150 EDs to FVIII products, 10 subjects received XYNTHA for on-demand and follow-up treatment. The median dose per on-demand infusion was 47 IU/kg (min-max: 24–74) and the median exposure per subject was 6 days (min-max: 1–26).
Of the 18 subjects <17 years of age who received at least 1 dose of XYNTHA, 10 subjects had bleeding episodes during the study. A total of 66 bleeding episodes were treated with on-demand infusions of XYNTHA. The majority of the bleeding episodes (63/66 or 95%) resolved with 1 or 2 infusions. The response to infusion was rated on a pre-specified 4 point hemostatic efficacy scale. Thirty-eight (38) of 66 bleeding episodes (58%) were rated excellent or good in their response to initial treatment, 24 (36%) were rated as moderate, and 4 (6%) were not rated.
Additional data for 50 subjects are available from a second safety and efficacy study of XYNTHA in children <16 years of age with severe to moderately severe hemophilia A (FVIII:C ≤2%) and with at least 20 prior EDs to FVIII products. Of the 50 subjects, 38 subjects received XYNTHA for on-demand and follow-up treatment of bleeding episodes. The median dose per on-demand infusion was 28 IU/kg (min-max: 10–92) and the median exposure per subject was 9 days (min-max: 1–95).
Of the 50 subjects <16 years of age who received at least 1 dose of XYNTHA, 38 had 562 bleeding episodes during the study. The majority of the bleeding episodes (518/562 or 92%) resolved with 1 or 2 infusions. Of 559 bleeding episodes treated with XYNTHA with response assessments to the first infusion, 526 (94%) were rated excellent or good in their response to initial treatment and 27 (5%) were rated as moderate.
In comparison to the pharmacokinetic parameters reported in adults, children have shorter half-lives, larger volumes of distribution and lower recovery of factor VIII after XYNTHA administration. The clearance (based on per kg body weight) is approximately 40% higher in children. Higher or more frequent doses may be required to account for the observed differences in pharmacokinetic parameters. [see Clinical Pharmacology (12.3)]
8.5 Geriatric Use
Clinical studies of XYNTHA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
-
11 DESCRIPTION
The active ingredient in XYNTHA, Antihemophilic Factor (Recombinant), is a recombinant antihemophilic factor (rAHF), also called coagulation factor VIII, which is produced by recombinant DNA technology. It is secreted by a genetically engineered Chinese hamster ovary (CHO) cell line. The cell line is grown in a chemically defined cell culture medium that contains recombinant insulin, but does not contain any materials derived from human or animal sources.
The rAHF in XYNTHA is a purified glycoprotein, with an approximate molecular mass of 170 kDa consisting of 1,438 amino acids, which does not contain the B-domain.13 The amino acid sequence of the rAHF is comparable to the 90 + 80 kDa form of human coagulation factor VIII.
The purification process uses a series of chromatography steps, one of which is based on affinity chromatography using a patented synthetic peptide affinity ligand.14 The process also includes a solvent-detergent viral inactivation step and a virus-retaining nanofiltration step.
The potency expressed in International Units (IU) is determined using the chromogenic assay of the European Pharmacopoeia. The Wyeth manufacturing reference standard for potency has been calibrated against the World Health Organization (WHO) International Standard for factor VIII activity using the one-stage clotting assay. The specific activity of XYNTHA is 5,500 to 9,900 IU per milligram of protein.
XYNTHA is formulated as a sterile, nonpyrogenic, no preservative, lyophilized powder preparation for intravenous injection. Each single-use vial contains nominally 250, 500, 1000, or 2000 IU of XYNTHA. Upon reconstitution, the product is a clear to slightly opalescent, colorless solution that contains sodium chloride, sucrose, L-histidine, calcium chloride, and polysorbate 80.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
XYNTHA temporarily replaces the missing clotting factor VIII that is needed for effective hemostasis.
12.2 Pharmacodynamics
The activated partial thromboplastin time (aPTT) is prolonged in patients with hemophilia. Determination of aPTT is a conventional in vitro assay for biological activity of factor VIII. Treatment with XYNTHA normalizes the aPTT over the effective dosing period.
12.3 Pharmacokinetics
The pharmacokinetic parameters of XYNTHA in 30 PTPs 12 to 60 years old, who received a single infusion of 50 IU/kg XYNTHA are summarized in Table 3.
In addition, 25 of the same subjects later received a single infusion of 50 IU/kg of XYNTHA for a 6-month follow-up pharmacokinetic study. The parameters were comparable between baseline and 6 months, indicating no time-dependent changes in the pharmacokinetics of XYNTHA.
In a separate study, 8 of 30 subjects at least 12 years old with hemophilia A undergoing elective major surgery received a single 50 IU/kg infusion of XYNTHA. The pharmacokinetic parameters in these subjects are also summarized in Table 3.
Table 3: Mean ± SD XYNTHA Pharmacokinetic Parameters in Previously Treated Patients with Hemophilia A after Single 50 IU/kg Dose Parameter Initial Visit
(n = 30)Month 6
(n = 25)Pre-surgery
(n=8)Abbreviations: AUC∞ = area under the plasma concentration-time curve from zero to infinity; Cmax = peak concentration; t1/2 = plasma elimination half-life; CL = clearance; n = number of subjects; SD = standard deviation; Vss = volume of distribution at steady-state. - * One subject was excluded from the calculation due to lack of a well-defined terminal phase.
Cmax (IU/mL) 1.08 ± 0.22 1.24 ± 0.42 1.08 ± 0.24 AUC∞ (IU∙hr/mL) 13.5 ± 5.6 15.0 ± 7.5 16.0 ± 5.2 t1/2 (hr) 11.2 ± 5.0 11.8 ± 6.2* 16.7 ± 5.4 CL (mL/hr/kg) 4.51 ± 2.23 4.04 ± 1.87 3.48 ± 1.25 Vss (mL/kg) 66.1 ± 33.0 67.4 ± 32.6 69.0 ± 20.1 Recovery
(IU/dL per IU/kg)2.15 ± 0.44 2.47 ± 0.84 2.17 ± 0.47 Table 4 shows the pharmacokinetic parameters of nine children; four aged 14 or 15 years of age, who are also included in the summary for the adults above, along with five children aged 3.7–5.8 years after single 50 IU/kg doses of XYNTHA. Compared to adults, the half-life of XYNTHA is shorter in children and the clearance (based on per kg body weight) is approximately 40% higher in children.
Table 4: Mean ± SD XYNTHA Pharmacokinetic Parameters in Previously Treated Pediatric Patients with Hemophilia A after Single 50 IU/kg Dose Parameter Young Children (n=5) Adolescents (n=4) Abbreviations: AUC∞ = area under the plasma concentration-time curve from zero to infinity; Cmax = peak concentration; t1/2 = plasma elimination half-life; CL = clearance; n = number of subjects; SD = standard deviation; Vss = volume of distribution at steady-state. Age (min - max, yr)) 3.7 – 5.8 14 – 15 Cmax (IU/mL) 0.78 ± 0.34 0.97 ± 0.21 AUC∞ (IU∙hr/mL) 12.2 ± 6.50 8.5 ± 4.0 t1/2 (hr) 8.3 ± 2.7 6.9 ± 2.4 CL (mL/hr/kg) 6.29 ± 4.87 6.62 ± 2.16 Vss (mL/kg) 66.9 ± 55.6 67.1 ± 13.6 Recovery
(IU/dL per IU/kg)1.52 ± 0.69 1.95 ± 0.41 -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted with XYNTHA to assess its mutagenic or carcinogenic potential. XYNTHA has been shown to be comparable to the predecessor product with respect to its biochemical and physicochemical properties, as well as its nonclinical in vivo pharmacology and toxicology. By inference, predecessor product and XYNTHA would be expected to have equivalent mutagenic and carcinogenic potential. The predecessor product has been shown to be nongenotoxic in the mouse micronucleus assay. No studies have been conducted in animals to assess impairment of fertility or fetal development.
13.2 Animal Toxicology and/or Pharmacology
Preclinical studies evaluating XYNTHA in hemophilia A dogs without inhibitors demonstrated safe and effective restoration of hemostasis. XYNTHA demonstrated a toxicological profile that was similar to the toxicological profile observed with the predecessor product. Toxicity associated with XYNTHA was primarily associated with anti-FVIII neutralizing antibody generation first detectable at 15 days of repeat dosing in high (approximately 735 IU/kg/day) level-dosed, non-human primates.
-
14 CLINICAL STUDIES
Two completed multicenter, open-label studies support the analysis of safety and efficacy of XYNTHA in on-demand treatment and control of bleeding episodes and perioperative management. These completed clinical studies for XYNTHA examined 124 PTP subjects, 94 for on-demand treatment and routine prophylaxis and 30 for surgical prophylaxis. Subjects with severe to moderately severe hemophilia A (FVIII:C ≤2%) and no history of FVIII inhibitors were eligible for the trials.
On-demand treatment and Control of Bleeding Episodes
Ninety-four (94) subjects, 12 years of age and older received XYNTHA in a routine prophylaxis treatment regimen with on-demand treatment administered as clinically indicated. All 94 subjects were treated with at least one dose and all are included in the intent-to-treat (ITT) population. Eighty-nine (89) subjects accrued ≥50 EDs. Median age for the 94 treated subjects was 24 years (mean 28 and min-max: 12–60 years).
Of these 94 subjects, 30 evaluable subjects participated in a randomized crossover pharmacokinetics substudy. Twenty-five (25/30) of these subjects with FVIII:C ≤1% completed both the first (PK1) and the second (PK2) pharmacokinetic assessments [see Clinical Pharmacology (12.3)].16
For routine prophylaxis, XYNTHA was administered at a dose of 30 ± 5 IU/kg 3 times a week with provisions for dose escalation based on pre-specified criteria. Seven dose escalations were prescribed for 6 subjects during the course of the study. Forty-three subjects (43/94 or 45.7%) reported no bleeding while on routine prophylaxis. The median annualized bleeding rate (ABR) for all bleeding episodes was 1.9 (mean 3.9, min-max: 0–42.1).
Fifty-three subjects (53/94) received XYNTHA on-demand treatment for a total of 187 bleeding episodes. Seven of these bleeding episodes occurred in subjects prior to switching to a prophylaxis treatment regimen. One hundred ten of 180 bleeds (110/180 or 61%) occurred ≤48 hours after the last dose and 39% (70/180 bleeds) occurred >48 hours after the last dose. The majority of bleeds reported to occur ≤48 hours after the last prophylaxis dose were traumatic (64/110 bleeds or 58%). Forty-two bleeds (42/70 or 60%) reported to occur >48 hours after the last prophylaxis dose were spontaneous. The on-demand treatment dosing regimen was determined by the investigator. The median dose for on-demand treatment was 31 IU/kg (min-max: 6–74 IU/kg) and the median exposure per subject was 3 days (min-max: 1–26).
The majority of bleeding episodes (173/187 or 93%) resolved with 1 or 2 infusions (Table 5). One hundred thirty-two of 187 bleeding episodes (132/187 or 71%) treated with XYNTHA were rated excellent or good in their response to initial treatment, 45 (24%) were rated moderate. Five (3%) were rated no response, and 5 (3%) were not rated.
Table 5: Summary of Response to Infusions to Treat New Bleeding Episode by Number of Infusions Needed for Resolution Number of Infusions (%) Response to 1st Infusion 1 2 3 4 >4 Total Number of Bleeds - * Excellent: Definite pain relief and/or improvement in signs of bleeding starting within 8 hours after an infusion, with no additional infusion administered.
- † Good: Definite pain relief and/or improvement in signs of bleeding starting within 8 hours after an infusion, with at least one additional infusion administered for complete resolution of the bleeding episode.
- ‡ Moderate: Probable or slight improvement starting after 8 hours following the infusion, with at least one additional infusion administered for complete resolution of the bleeding episode.
- § No Response: No improvement at all between infusions or during the 24 hour interval following an infusion, or condition worsens.
- ¶ Includes one infusion with commercial FVIII that occurred before routine prophylaxis began.
Excellent* 42 (95.5) 2 (4.5) 0 (0.0) 0 (0.0) 0 (0.0) 44 Good† 69 (78.4) 16 (18.2) 3 (3.4) 0 (0.0) 0 (0.0) 88 Moderate‡ 24 (53.3) 16 (35.6) 2 (4.4) 0 (0.0) 3 (6.7) 45 No Response§ 0 (0.0) 0 (0.0) 2 (40.0) 2 (40.0) 1 (20.0) 5 Not Assessed 4 (80.0) 0 (0.0) 0 (0.0) 1 (20.0) 0 (0.0) 5¶ Total 139 (74.3) 34 (18.2) 7 (3.7) 3 (1.6) 4 (2.1) 187 Perioperative Management
In a study (n=30) for surgical prophylaxis in subjects with hemophilia A, XYNTHA was administered to 25 efficacy-evaluable PTPs undergoing major surgical procedures (11 total knee replacements, 1 hip replacement, 5 synovectomies, 1 left ulnar nerve transposition release, 1 ventral hernia repair/scar revision, 1 knee arthroscopy, 1 revision and debridement of the knee after a total knee replacement, 1 hip arthroplasty revision, 1 stapes replacement, 1 ankle arthrodesis, and 1 pseudotumor excision).17
The results of the hemostatic efficacy ratings for these subjects are presented in Table 6. Investigator's ratings of efficacy at the end of surgery and at the end of the initial postoperative period were excellent or good for all assessments. Intraoperative blood loss was reported as "normal" or "absent" for all subjects. Thirteen of the subjects (13/25 or 52%) had blood loss in the postoperative period. The postoperative blood loss was rated as "normal" for ten of these cases while three cases were rated "abnormal" (1 due to hemorrhage following surgical trauma to the epigastric artery, 1 due to an 800 mL blood loss after hip replacement surgery, and 1 after an elbow synovectomy where the blood loss could not be measured by the investigator).
Table 6: Summary of Hemostatic Efficacy Time of Hemostatic Efficacy Assessment Excellent* Good† Number of subjects - * Excellent : Achieved hemostasis comparable to that expected after similar surgery in a patient without hemophilia.
- † Good: Prolonged time to hemostasis, with somewhat increased bleeding compared with that expected after similar surgery in a patient without hemophilia.
- ‡ End of initial postoperative period is date of discharge or postoperative Day 6, whichever occurs later.
End of surgery 18 (72%) 7 (28%) 25 End of initial postoperative period‡ 23 (92%) 2 (8%) 25 -
15 REFERENCES
- Nilsson IM, Berntorp EE and Freiburghaus C. Treatment of patients with factor VIII and IX inhibitors. Thromb Haemost. 1993;70(1):56–59.
- Hoyer LW. Hemophilia A. N Engl J Med. 1994;330:38–47.
- Juhlin F. Stability and Compatibility of Reconstituted Recombinant Factor VIII SQ, 250 IU/ml, in a System for Continuous Infusion. Pharmacia Document 9610224, 1996.
- Ehrenforth S, Kreuz W, Scharrer I, et al. Incidence of development of factor VIII and factor IX inhibitors in hemophiliacs. Lancet. 1992;339:594–598.
- Lusher J, Arkin S, Abildgaard CF, Schwartz RS, the Kogenate PUP Study Group. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. N Engl J Med. 1993;328:453–459.
- Bray GL, Gomperts ED, Courter S, et al. A multicenter study of recombinant factor VIII (Recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. Blood. 1994;83(9):2428–2435.
- Kessler C, Sachse K. Factor VIII:C inhibitor associated with monoclonal-antibody purified FVIII concentrate. Lancet. 1990;335:1403.
- Schwartz RS, Abildgaard CF, Aledort LM, et al. Human recombinant DNA-derived antihemophilic factor (factor VIII) in the treatment of hemophilia A. N Engl J Med. 1990;323:1800–1805.
- White GC II, Courter S, Bray GL, et al. A multicenter study of recombinant factor VIII (Recombinate™) in previously treated patients with hemophilia A. Thromb Haemost. 1997;77(4):660–667.
- Gruppo R, Chen H, Schroth P, et al. Safety and immunogenicity of recombinant factor VIII (Recombinate™) in previously untreated patients: A 7.3 year update. Haemophilia. 1998;4:228 (Abstract No. 291, XXIII Congress of the WFH, The Hague).
- Scharrer I, Bray GL, Neutzling O. Incidence of inhibitors in haemophilia A patients - a review of recent studies of recombinant and plasma-derived factor VIII concentrates. Haemophilia. 1999;5:145–154.
- Abshire TC, Brackmann HH, Scharrer I, et al. Sucrose formulated recombinant human antihemophilic Factor VIII is safe and efficacious for treatment of hemophilia A in home therapy: Results of a multicenter, international, clinical investigation. Thromb Haemost. 2000;83(6):811–816.
- Sandberg H, Almstedt A, Brandt J, Castro VM, Gray E, Holmquist L, et al. Structural and Functional Characterization of B-Domain Deleted Recombinant Factor VIII. Sem Hematol. 2001;38 (Suppl. 4):4–12.
- Kelley BD, Tannatt M, Magnusson R, Hagelberg S. Development and Validation of an Affinity Chromatography Step Using a Peptide Ligand for cGMP Production of Factor VIII. Biotechnol Bioeng. 2004;87(3):400–412.
- Mann KG and Ziedens KB. Overview of Hemostasis. In: Lee CA, Berntorp EE and Hoots WK, eds. Textbook of Hemophilia. USA, Blackwell Publishing; 2005:1–4.
- Recht M, Nemes L, Matysiak M et al. Clinical Evaluation of Moroctocog Alfa (AF-CC), a New Generation of B-Domain Deleted Recombinant Factor VIII (BDDrFVIII) for Treatment of Haemophilia A: Demonstration of Safety, Efficacy and Pharmacokinetic Equivalence to Full-length Recombinant Factor VIII. Haemophilia 2009; 1–12.
- Windyga J, Rusen L, Gruppo R, et al. BDDrFVIII (Moroctocog alfa [AF-CC]) for surgical haemostasis in patients with haemophilia A: results of a pivotal study. Haemophilia. 2010 Sep 1;16(5):731–9.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
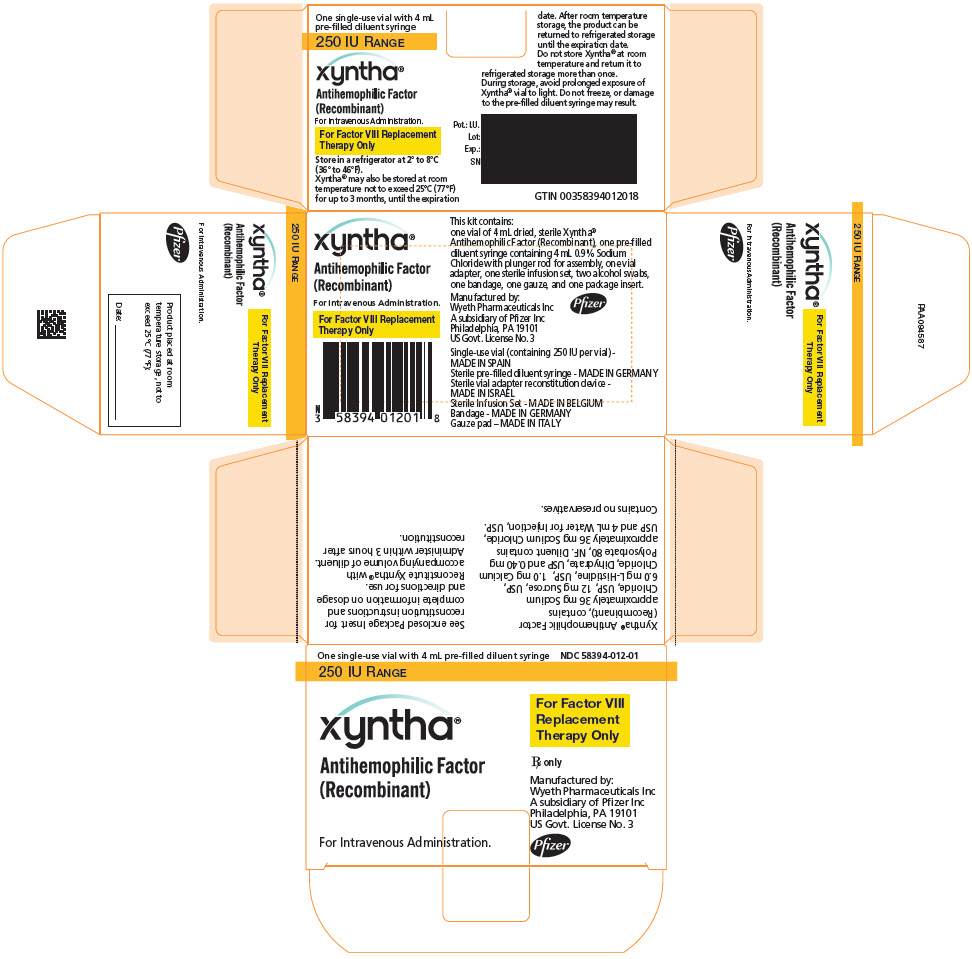
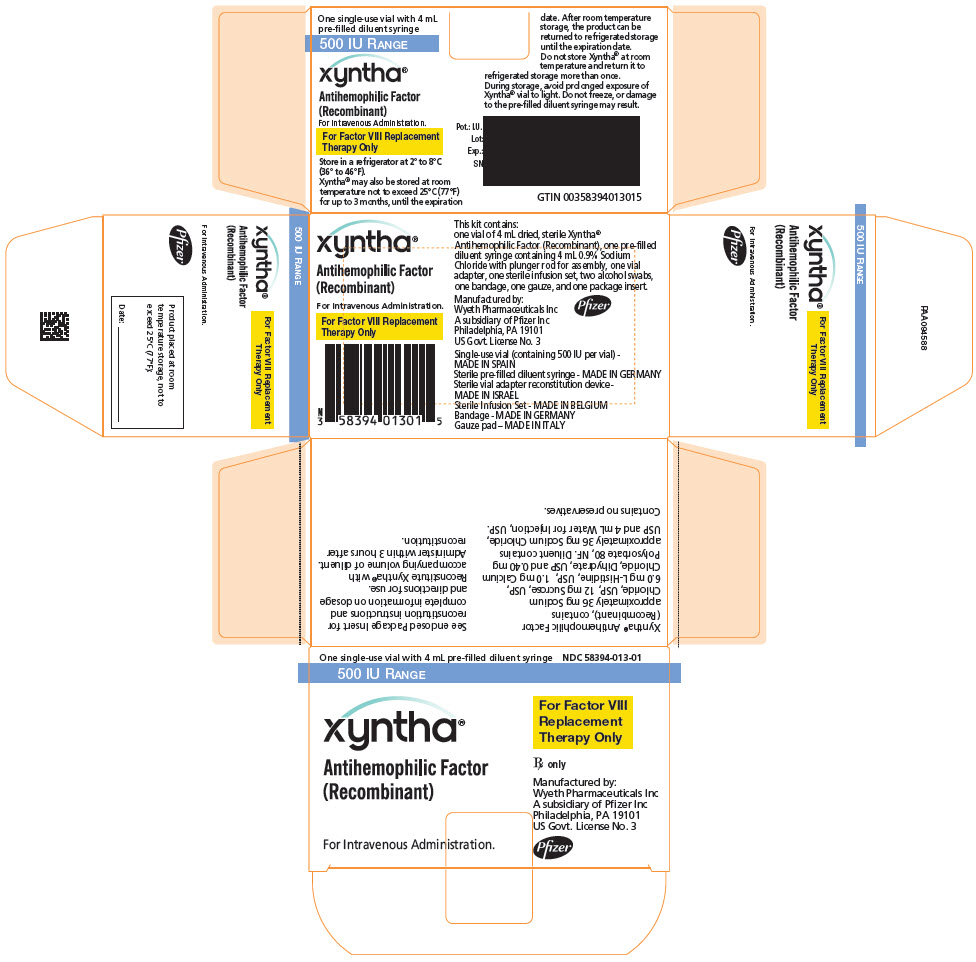
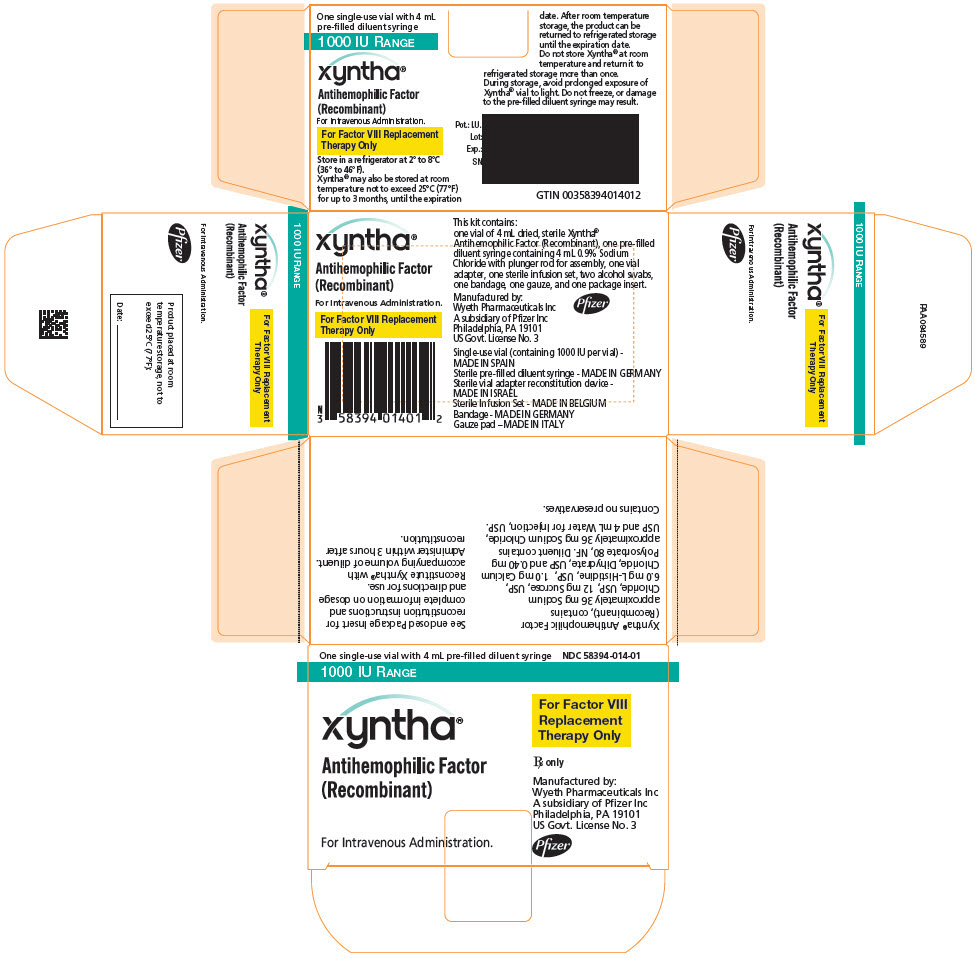
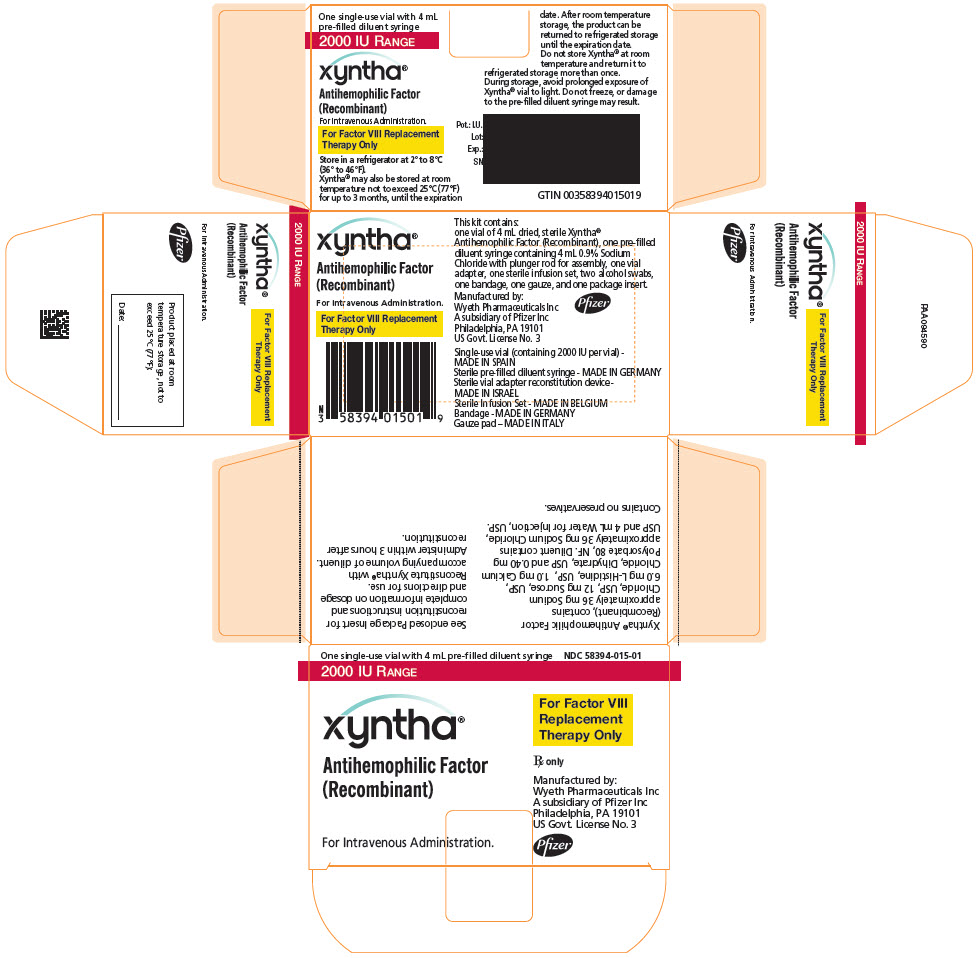
Each XYNTHA Vial Kit contains: one prefilled diluent syringe containing 4 mL 0.9% Sodium Chloride with plunger rod for assembly, one vial adapter, one sterile infusion set, two alcohol swabs, one bandage, one gauze pad, and one package insert. The drug product, diluents for injection and the rest of components included within the XYNTHA 250, 500, 1000, or 2000 International Units kit are free from natural rubber and natural rubber latex.
XYNTHA is supplied in kits that include single-use vials containing nominally 250, 500, 1000, or 2000 International Units lyophilized powder per vial:
XYNTHA Vial Kit Nominal Strength Fill Size Color Indicator Vial Kit NDC # 250 International Units Yellow 58394-012-01 500 International Units Blue 58394-013-01 1000 International Units Green 58394-014-01 2000 International Units Red 58394-015-01 Actual factor VIII activity in International Units is stated on the label of each XYNTHA vial.
Storage and Handling
- Store XYNTHA under refrigeration at a temperature of 2° to 8°C (36° to 46°F) for up to 36 months from the date of manufacture until the expiration date stated on the label.
- Within the expiration date, XYNTHA also may be stored at room temperature not to exceed 25°C (77°F) for up to 3 months.
- After room temperature storage, XYNTHA can be returned to the refrigerator until the expiration date. Do not store XYNTHA at room temperature and return it to the refrigerator more than once.
- Clearly record the starting date at room temperature storage in the space provided on the outer carton. At the end of the 3-month period, immediately use, discard, or return the product to refrigerated storage. The diluent syringe may be stored at 2° to 25°C (36° to 77°F).
- Do not use XYNTHA after the expiration date.
- Do not freeze. (Freezing may damage the prefilled diluent syringe.)
- During storage, avoid prolonged exposure of XYNTHA vial to light.
- Store the reconstituted solution at room temperature prior to administration. Administer XYNTHA within 3 hours after reconstitution.
-
17 PATIENT COUNSELING INFORMATION
- Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Advise patients to report any adverse reactions or problems that concern them when taking XYNTHA to their healthcare provider.
- Allergic-type hypersensitivity reactions are possible. Discuss the early signs of hypersensitivity reactions (including hives [rash with itching]), generalized urticaria, tightness of the chest, wheezing, hypotension) and anaphylaxis. Advise patients to discontinue use of the product, call their healthcare provider, and go to the emergency department if these symptoms occur.
- Advise patients to contact their healthcare provider if they experience a lack of a clinical response to factor VIII replacement therapy, as this may be a manifestation of an inhibitor.
- Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during therapy, or if they are breastfeeding.
-
FDA-Approved Patient Labeling
Patient Information
XYNTHA® /ZIN-tha/
[Antihemophilic Factor (Recombinant)]Please read this patient information carefully before using XYNTHA and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical problems or your treatment.
What is XYNTHA?
XYNTHA is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia A. Hemophilia A is also called classic hemophilia. Your healthcare provider may give you XYNTHA when you have surgery.
XYNTHA is not used to treat von Willebrand's disease.
What should I tell my healthcare provider before using XYNTHA?
Tell your healthcare provider about all of your medical conditions, including if you:
- have any allergies, including allergies to hamsters.
- are pregnant or planning to become pregnant. It is not known if XYNTHA may harm your unborn baby.
- are breastfeeding. It is not known if XYNTHA passes into your milk and if it can harm your baby.
Tell your healthcare provider about all of the medicines you take, including all prescription and non-prescription medicines, such as over-the-counter medicines, supplements, or herbal remedies.
How should I infuse XYNTHA?
Step-by-step instructions for infusing with XYNTHA are provided at the end of this leaflet.
The steps listed below are general guidelines for using XYNTHA. Always follow any specific instructions from your healthcare provider. If you are unsure of the procedures, please call your healthcare provider before using.
Call your healthcare provider right away if bleeding is not controlled after using XYNTHA.
Call your healthcare provider right away if you take more than the dose you should take.
Talk to your healthcare provider before traveling. Plan to bring enough XYNTHA for your treatment during this time.
What are the possible side effects of XYNTHA?
Call your healthcare provider or go to the emergency department right away if you have any of the following symptoms because these may be signs of a serious allergic reaction:
- wheezing
- difficulty breathing
- chest tightness
- turning blue (look at lips and gums)
- fast heartbeat
- swelling of the face
- faintness
- rash
- hives
Common side effects of XYNTHA are
- headache
- fever
- nausea
- vomiting
- diarrhea
- weakness
Your body can make antibodies against XYNTHA (called "inhibitors") that may stop XYNTHA from working properly. Your healthcare provider may need to take blood tests from time to time to monitor for inhibitors.
Talk to your healthcare provider about any side effect that bothers you or that does not go away. You may report side effects to FDA at 1-800-FDA-1088.
How should I store XYNTHA?
Store XYNTHA in the refrigerator at 36° to 46°F (2° to 8°C). Store the diluent syringe at 36° to 77°F (2° to 25°C).
Do not freeze.
Protect from light.
XYNTHA can last at room temperature (below 77°F) for up to 3 months. If you store XYNTHA at room temperature, carefully write down the date you put XYNTHA at room temperature, so you will know when to either put it back in the refrigerator, use it immediately, or throw it away. There is a space on the carton for you to write the date.
If stored at room temperature, XYNTHA can be returned one time to the refrigerator until the expiration date. Do not store at room temperature and return it to the refrigerator more than once. Throw away any unused XYNTHA after the expiration date.
Infuse XYNTHA within 3 hours of reconstitution. You can keep the reconstituted solution at room temperature before infusion for up to 3 hours. If you have not used it in 3 hours, throw it away.
Do not use reconstituted XYNTHA if it is not clear to slightly opalescent and colorless.
Dispose of all materials, whether reconstituted or not, in an appropriate medical waste container.
What else should I know about XYNTHA?
Medicines are sometimes prescribed for purposes other than those listed here. Talk to your healthcare provider if you have any concerns. You can ask your healthcare provider for information about XYNTHA that was written for healthcare professionals.
Do not share XYNTHA with other people, even if they have the same symptoms that you have.
-
Instructions for Use
XYNTHA® /ZIN-tha/
[Antihemophilic Factor (Recombinant)]XYNTHA is supplied as a lyophilized powder. Before you can infuse it (intravenous injection), you must reconstitute the powder by mixing it with the liquid diluent supplied. The liquid diluent is 0.9% sodium chloride.
Reconstitute and infuse XYNTHA using the infusion set, diluent, syringe, and adapter provided in this kit. Please follow the directions below for the proper use of this product.
PREPARATION AND RECONSTITUTION OF XYNTHA
Preparation
- Always wash your hands before doing the following steps.
- Keep everything clean and germ-free while you are reconstituting XYNTHA.
- Once the vials are open, finish reconstituting XYNTHA as soon as possible. This will help to keep them germ-free.
- For additional instructions on the use of a XYNTHA Vial Kit and a XYNTHA SOLOFUSE kit, see detailed information provided after the INFUSION OF XYNTHA section.
Reconstitution
Note: If you use more than one vial of XYNTHA for each infusion, reconstitute each vial according to steps 1 through 11.
- Let the XYNTHA vial and the prefilled diluent syringe reach room temperature.
- Remove the plastic flip-top cap from the XYNTHA vial to show the center part of the rubber stopper.
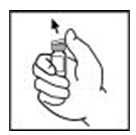
- Wipe the top of the vial with the alcohol swab provided, or use another antiseptic solution, and allow to dry. After cleaning, do not touch the rubber stopper with your hand or allow it to touch any surface.
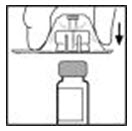
- Peel back the cover from the clear plastic vial adapter package. Do not remove the adapter from the package.
- Place the XYNTHA vial on a flat surface. While holding the adapter in the package, place the vial adapter over the XYNTHA vial. Press down firmly on the package until the adapter snaps into place on top of the vial, with the adapter spike going into the vial stopper.
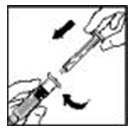
- Grasp the plunger rod as shown in the picture below. Do not touch the shaft of the plunger rod. Attach the threaded end of the plunger rod to the diluent syringe plunger by pushing and turning firmly.
- Break off the tamper-resistant, plastic tip cap from the diluent syringe by snapping the perforation of the cap. Do not touch the inside of the cap or the syringe tip. The diluent syringe may need to be recapped (if reconstituted XYNTHA is not used immediately), so place the cap on its top on a clean surface in a spot where it will stay clean.
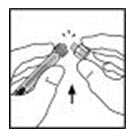
- Lift the package cover away from the adapter and throw the package away.
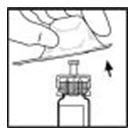
- Place the XYNTHA vial on a flat surface. Connect the diluent syringe to the vial adapter by inserting the tip of the syringe into the adapter opening while firmly pushing and turning the syringe clockwise until the connection is secured.
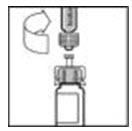
- Slowly push the plunger rod to inject all the diluent into the XYNTHA vial.
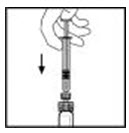
- With the syringe still connected to the adapter, gently swirl the contents of the vial until the powder is dissolved.
Look carefully at the final solution. The solution should be clear to slightly opalescent and colorless. If it is not, throw away the solution and use a new kit. - Make sure the syringe plunger rod is still fully pressed down, then turn over the XYNTHA vial. Slowly pull the solution into the syringe. Turn the syringe upward again and remove any air bubbles by gently tapping the syringe with your finger and slowly pushing air out of the syringe.
If you reconstituted more than one vial of XYNTHA, remove the diluent syringe from the vial adapter and leave the vial adapter attached to the XYNTHA vial. Quickly attach a separate large luer lock syringe and pull the reconstituted solution as instructed above. Repeat this procedure with each vial in turn. Do not detach the diluent syringe or the large luer lock syringe until you are ready to attach the large luer lock syringe to the next vial adapter.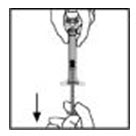
- Remove the syringe from the vial adapter by gently pulling and turning the syringe counterclockwise. Throw away the empty XYNTHA vial with the adapter attached.
Note:
- If you are not using the solution right away, carefully replace the syringe cap. Do not touch the syringe tip or the inside of the cap.
- Infuse XYNTHA solution within 3 hours after reconstitution. The reconstituted solution may be kept at room temperature for up to 3 hours prior to infusion. If you have not used it in 3 hours, throw it away.
INFUSION OF XYNTHA
Your healthcare provider will teach you how to infuse XYNTHA yourself. Once you learn how to do this, you can follow the instructions in this insert.
Before XYNTHA can be infused, you must reconstitute it as instructed above in the PREPARATION AND RECONSTITUTION OF XYNTHA section.
After reconstitution, be sure to look carefully at the XYNTHA solution. The solution should be clear to slightly opalescent and colorless. If it is not, throw away the solution and use a new kit.
Use the infusion set included in the kit to infuse XYNTHA. Do not infuse XYNTHA in the same tubing or container with other medicines.
- Attach the syringe to the luer end of the provided infusion set tubing.
- Apply a tourniquet and prepare the injection site by wiping the skin well with an alcohol swab provided in the kit.
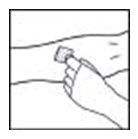
- Remove the protective needle cover and insert the butterfly needle of the infusion set tubing into your vein as instructed by your healthcare provider. Remove the tourniquet. Verify proper needle placement.
- Infuse the reconstituted XYNTHA product over several minutes. Your comfort level should determine the rate of infusion.
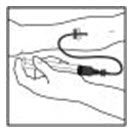
- After infusing XYNTHA, remove the infusion set and throw it away. The amount of liquid left in the infusion set will not affect your treatment.
Note:
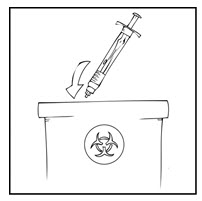
- Dispose of all unused solution, the empty vial(s), and other used medical supplies in an appropriate container.
- It is a good idea to record the lot number from the XYNTHA vial label every time you use XYNTHA. You can use the peel-off label found on the vial to record the lot number.
ADDITIONAL INSTRUCTIONS
XYNTHA is also supplied in kits that have both the XYNTHA powder and the diluent within single-use prefilled dual-chamber syringes, called XYNTHA SOLOFUSE.
If you use one XYNTHA vial and one of XYNTHA SOLOFUSE for the infusion, reconstitute the XYNTHA vial and the XYNTHA SOLOFUSE according to the specific directions for that respective product kit. Use a separate 10 milliliter or larger luer lock syringe (not included in this kit) to draw back the reconstituted contents of the XYNTHA vial and XYNTHA SOLOFUSE.
Use of a XYNTHA Vial Kit with a XYNTHA SOLOFUSE Kit
These instructions are for the use of only one XYNTHA vial kit and one XYNTHA SOLOFUSE Kit. For further information, please contact your healthcare provider or call the Medical Information Department at Wyeth Pharmaceuticals, 1-800-438-1985.
- Reconstitute the XYNTHA vial using the instructions described in PREPARATION AND RECONSTITUTION OF XYNTHA section. Detach the empty diluent syringe from the vial adapter by gently turning and pulling the syringe counterclockwise, leaving the contents in the XYNTHA vial with the vial adapter in place.
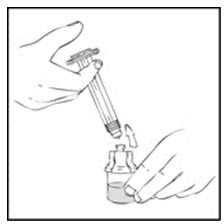
- Reconstitute the XYNTHA SOLOFUSE using the instructions included with the kit, remembering to remove most, but not all, of the air from the syringe.
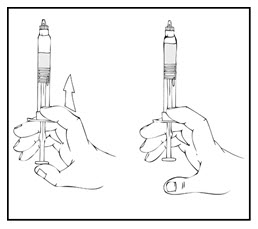
- After removing the protective blue vented cap, connect the XYNTHA SOLOFUSE to the vial adapter by inserting the tip into the adapter opening while firmly pushing and turning the syringe clockwise until secured.
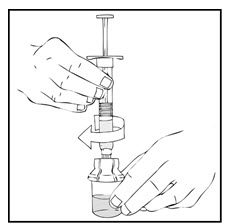
- Slowly depress the plunger rod of the XYNTHA SOLOFUSE until the contents empty into the XYNTHA vial. The plunger rod may move back slightly after release.
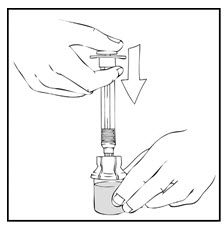
- Detach the empty XYNTHA SOLOFUSE from the vial adapter and throw it away.
If the syringe turns without detaching from the vial adapter, grasp the white collar and turn.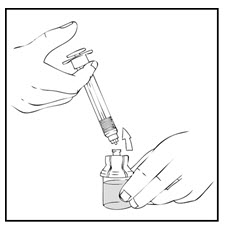
- Connect a sterile 10 milliliter or larger luer lock syringe to the vial adapter. You may want to inject some air into the XYNTHA vial to make withdrawing the vial contents easier.
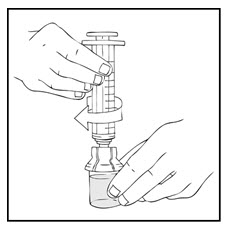
- Invert the XYNTHA vial and slowly draw the solution into the large luer lock syringe.
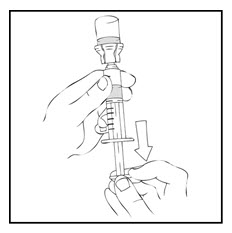
- Detach the large luer lock syringe from the vial adapter by gently turning and pulling the syringe counterclockwise. Throw away the empty XYNTHA vial with the adapter attached.
- Attach the infusion set to the large luer lock syringe as directed in the INFUSION OF XYNTHA section.
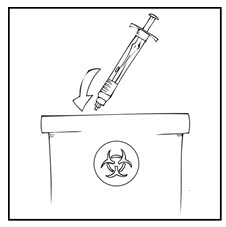
Note: Dispose of all unused solution and other used medical supplies in an appropriate container.
This product's label may have been updated. For current full prescribing information, please visit www.pfizer.com.

License no: 3
LAB-0516-7.0 -
PRINCIPAL DISPLAY PANEL - 4 mL Syringe Label
0.9% Sodium
Chloride Diluent,
4 mLDisposable syringe for
drug diluent use with Xyntha®
Antihemophilic Factor
(Recombinant) Sterile,
nonpyrogenic.
Contains no preservatives.Manufactured for:
Wyeth Pharmaceuticals LLC
A subsidiary of Pfizer Inc
Philadelphia, PA 19101
Manufactured by:
Vetter Pharma-Fertigung GmbH & Co. KG
88212 Ravensburg, Germany
or
Vetter Pharma-Fertigung GmbH & Co. KG
Eisenbahnstrasse 2-4 88085 Langenargen,
GermanyPfizer
Rx only
US Govt. License No. 3
LOT:
EXP:
PAA134633
-
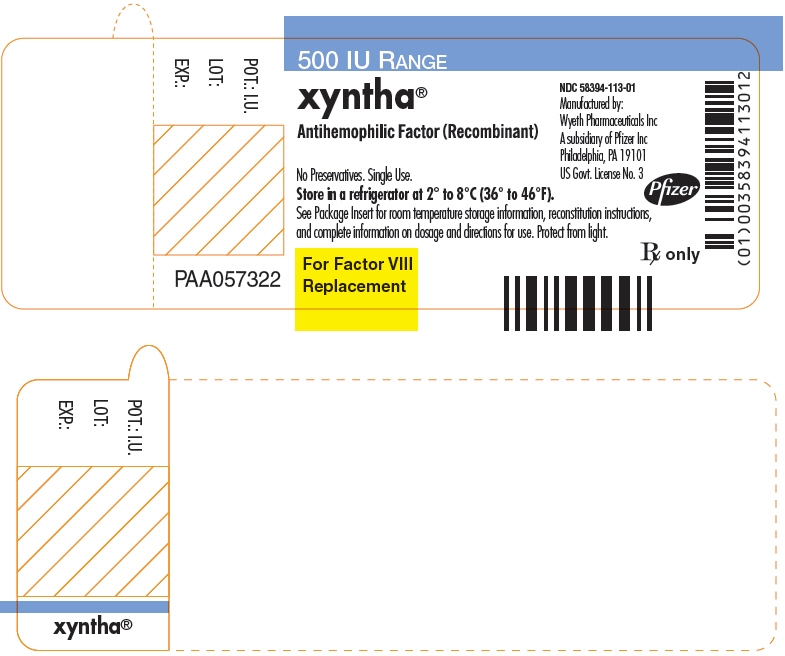
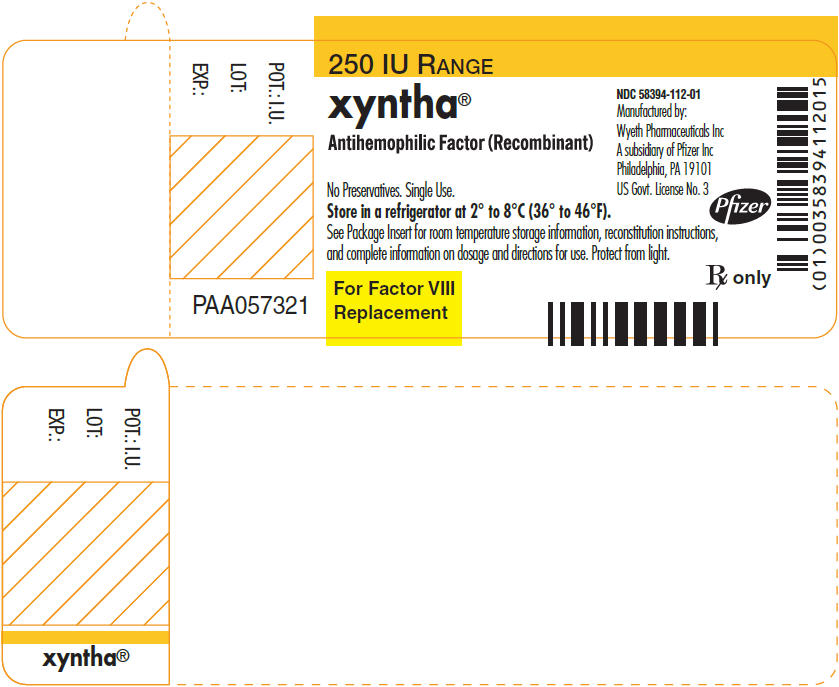
PRINCIPAL DISPLAY PANEL - 250 IU Label
250 IU RANGE
xyntha®
Antihemophilic Factor (Recombinant)
No Preservatives. Single Use.
Store in a refrigerator at 2° to 8°C (36° to 46°F).
See Package Insert for room temperature storage information, reconstitution instructions,
and complete information on dosage and directions for use. Protect from light.For Factor VIII
ReplacementNDC: 58394-112-01
Manufactured by:
Wyeth Pharmaceuticals Inc
A subsidiary of Pfizer Inc
Philadelphia, PA 19101
US Govt. License No. 3Pfizer
Rx only
POT.: I.U.
LOT:
EXP.:
PAA057321
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 250 IU
One single-use vial with 4 mL pre-filled diluent syringe
NDC: 58394-012-01250 IU RANGE
xyntha®
Antihemophilic Factor
(Recombinant)For Intravenous Administration.
For Factor VIII
Replacement
Therapy OnlyRx only
Manufactured by:
Wyeth Pharmaceuticals Inc
A subsidiary of Pfizer Inc
Philadelphia, PA 19101
US Govt. License No. 3Pfizer
- PRINCIPAL DISPLAY PANEL - 500 IU Vial Label
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 500 IU
One single-use vial with 4 mL pre-filled diluent syringe
NDC: 58394-013-01500 IU RANGE
xyntha®
Antihemophilic Factor
(Recombinant)For Intravenous Administration.
For Factor VIII
Replacement
Therapy OnlyRx only
Manufactured by:
Wyeth Pharmaceuticals LLC
A subsidiary of Pfizer Inc
Philadelphia, PA 19101
US Govt. License No. 3Pfizer
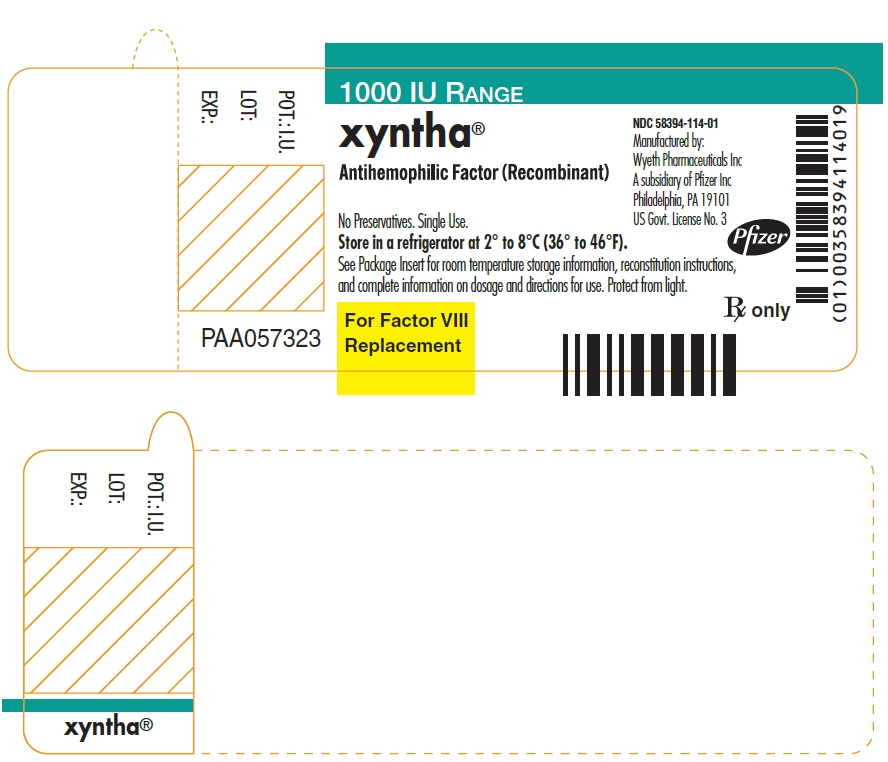
- PRINCIPAL DISPLAY PANEL - 1000 IU Vial Label
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 1000 IU
One single-use vial with 4 mL pre-filled diluent syringe
NDC: 58394-014-011000 IU RANGE
xyntha®
Antihemophilic Factor
(Recombinant)For Intravenous Administration.
For Factor VIII
Replacement
Therapy OnlyRx only
Manufactured by:
Wyeth Pharmaceuticals LLC
A subsidiary of Pfizer Inc
Philadelphia, PA 19101
US Govt. License No. 3Pfizer
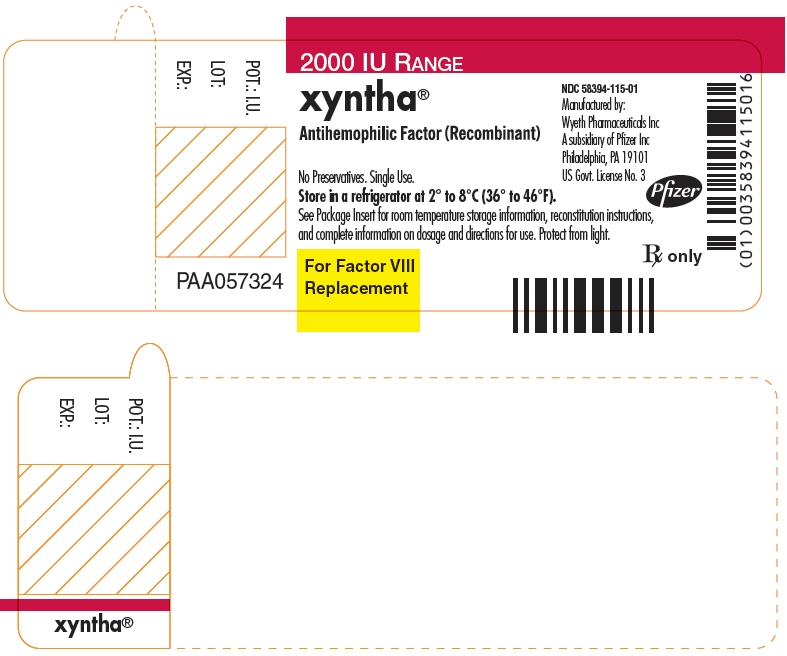
- PRINCIPAL DISPLAY PANEL - 2000 IU Vial Label
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 2000 IU
One single-use vial with 4 mL pre-filled diluent syringe
NDC: 58394-015-012000 IU RANGE
xyntha®
Antihemophilic Factor
(Recombinant)For Intravenous Administration.
For Factor VIII
Replacement
Therapy OnlyRx only
Manufactured by:
Wyeth Pharmaceuticals LLC
A subsidiary of Pfizer Inc
Philadelphia, PA 19101
US Govt. License No. 3Pfizer
-
INGREDIENTS AND APPEARANCE
XYNTHA
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 58394-012 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-012-01 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 4 mL Part 2 1 SYRINGE 4 mL Part 3 2 PACKET 2 mL Part 1 of 3 XYNTHA
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 58394-112 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOROCTOCOG ALFA (UNII: 113E3Z3CJJ) (MOROCTOCOG ALFA - UNII:113E3Z3CJJ) MOROCTOCOG ALFA 250 [iU] in 4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCROSE (UNII: C151H8M554) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-112-01 4 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 Part 2 of 3 DILUENT
diluent injection, solutionProduct Information Item Code (Source) NDC: 58394-018 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.036 mg in 4 mL WATER (UNII: 059QF0KO0R) 4 mL in 4 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-018-01 4 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 Part 3 of 3 ALCOHOL
alcohol swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part333A 08/01/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 XYNTHA
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 58394-013 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-013-01 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 4 mL Part 2 1 SYRINGE 4 mL Part 3 2 PACKET 2 mL Part 1 of 3 XYNTHA
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 58394-113 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOROCTOCOG ALFA (UNII: 113E3Z3CJJ) (MOROCTOCOG ALFA - UNII:113E3Z3CJJ) MOROCTOCOG ALFA 500 [iU] in 4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCROSE (UNII: C151H8M554) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-113-01 4 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 Part 2 of 3 DILUENT
diluent injection, solutionProduct Information Item Code (Source) NDC: 58394-018 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.036 mg in 4 mL WATER (UNII: 059QF0KO0R) 4 mL in 4 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-018-01 4 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 Part 3 of 3 ALCOHOL
alcohol swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part333A 08/01/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 XYNTHA
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 58394-014 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-014-01 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 4 mL Part 2 1 SYRINGE 4 mL Part 3 2 PACKET 2 mL Part 1 of 3 XYNTHA
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 58394-114 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOROCTOCOG ALFA (UNII: 113E3Z3CJJ) (MOROCTOCOG ALFA - UNII:113E3Z3CJJ) MOROCTOCOG ALFA 1000 [iU] in 4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCROSE (UNII: C151H8M554) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-114-01 4 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 Part 2 of 3 DILUENT
diluent injection, solutionProduct Information Item Code (Source) NDC: 58394-018 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.036 mg in 4 mL WATER (UNII: 059QF0KO0R) 4 mL in 4 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-018-01 4 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 Part 3 of 3 ALCOHOL
alcohol swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part333A 08/01/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 XYNTHA
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 58394-015 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-015-01 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 4 mL Part 2 1 SYRINGE 4 mL Part 3 2 PACKET 2 mL Part 1 of 3 XYNTHA
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 58394-115 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOROCTOCOG ALFA (UNII: 113E3Z3CJJ) (MOROCTOCOG ALFA - UNII:113E3Z3CJJ) MOROCTOCOG ALFA 2000 [iU] in 4 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCROSE (UNII: C151H8M554) HISTIDINE (UNII: 4QD397987E) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-115-01 4 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 Part 2 of 3 DILUENT
diluent injection, solutionProduct Information Item Code (Source) NDC: 58394-018 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.036 mg in 4 mL WATER (UNII: 059QF0KO0R) 4 mL in 4 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58394-018-01 4 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 Part 3 of 3 ALCOHOL
alcohol swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part333A 08/01/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125264 08/01/2008 Labeler - Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC (174350868) Establishment Name Address ID/FEI Business Operations Wyeth Farma SA 462005232 ANALYSIS(58394-012, 58394-013, 58394-014, 58394-015) , LABEL(58394-012, 58394-013, 58394-014, 58394-015) , MANUFACTURE(58394-012, 58394-013, 58394-014, 58394-015) , PACK(58394-012, 58394-013, 58394-014, 58394-015)
Trademark Results [Xyntha]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 XYNTHA 78754885 3535448 Live/Registered |
WYETH LLC 2005-11-16 |
 XYNTHA 77380592 3607161 Live/Registered |
WYETH LLC 2008-01-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.