GOLYTELY- polyethylene glycol 3350, sodium sulfate anhydrous, sodium bicarbonate, sodium chloride, potassium chloride powder, for solution
GoLYTELY by
Drug Labeling and Warnings
GoLYTELY by is a Prescription medication manufactured, distributed, or labeled by Braintree Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GoLYTELY safely and effectively. See full prescribing information for GoLYTELY.
GoLYTELY (polyethylene glycol 3350 and electrolytes oral solution)
Initial U.S. Approval: 1984RECENT MAJOR CHANGES
Warnings and Precautions ( 5) 9/2013
INDICATIONS AND USAGE
GoLYTELY is a combination of PEG 3350, an osmotic laxative, and electrolytes indicated for cleansing of the colon in preparation for colonoscopy and barium enema X-ray examination in adults ( 1)
DOSAGE AND ADMINISTRATION
- GoLYTELY, supplied as a powder, must be reconstituted with water before its use ( 2.1, 5.8)
- On day prior to colonoscopy, instruct patients to:
- Eat a light breakfast or have clear liquids (avoid red and purple liquids) ( 2.2).
- Early in the evening prior to colonoscopy, fill container containing GoLYTELY powder with lukewarm water to 4 liter fill line ( 2.2).
- After capping container, shake vigorously several times. Instruct patients to consume water or clear liquids during and after bowel preparation up until 2 hours before time of colonoscopy ( 2.3).
- Adults: Drink at a rate of 240 mL (8 oz.) every 10 minutes, until 4 liters are consumed or rectal effluent is clear. For nasogastric tube (NGT), rate is 1.2 to 1.8 liters per hour ( 2.3)
DOSAGE FORMS AND STRENGTHS
For oral solution: polyethylene glycol 3350 236 grams, sodium sulfate (anhydrous) 22.74 grams, sodium bicarbonate 6.74 grams, sodium chloride 5.86 grams, potassium chloride 2.97 grams, and flavoring ingredients 3 grams; supplied in one 4 liter disposable jug ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risk of fluid and electrolyte abnormalities, arrhythmias, seizures and renal impairment– assess concurrent medications and consider testing in some patients ( 5.1, 5.2, 5.3, 5.4)
- Patients with renal insufficiency– use caution, ensure adequate hydration and consider testing ( 5.4)
- Suspected GI obstruction or perforation – rule out the diagnosis before administration ( 4, 5.6)
- Patients at risk for aspiration – observe during administration ( 5.7)
- Not for direct ingestion – dilute and take with additional water ( 5.8)
ADVERSE REACTIONS
Most common adverse reactions (≥3%) are: nausea, abdominal fullness and bloating. Abdominal cramps, vomiting and anal irritation occur less frequently ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Braintree Laboratories, Inc. at 1-800-874-6756 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2013
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Overview
2.2 Administration Instructions Prior to Dosage
2.3 Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Serum Chemistry Abnormalities
5.2 Cardiac Arrhythmias
5.3 Seizures
5.4 Renal Impairment
5.5 Colonic Mucosal Ulcerations and Ischemic Colitis
5.6 Use in Patients with Significant Gastrointestinal Disease
5.7 Aspiration
5.8 Not for Direct Ingestion
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Drugs that May Lead to Fluid and Electrolyte Abnormalities
7.2 Potential for Altered Drug Absorption
7.3 Stimulant Laxatives
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Overview
GoLYTELY, supplied as a powder, must be reconstituted with water before its use; it is not for direct ingestion [see Dosage and Administration ( 2.2), Warnings and Precautions ( 5.8)] . The 4 liter reconstituted GoLYTELY solution contains: 236 grams of polyethylene glycol (PEG) 3350, 22.74 grams sodium sulfate (anhydrous), 6.74 grams of sodium bicarbonate, 5.86 grams of sodium chloride, and 2.97 grams of potassium chloride. GoLYTELY is also available with pineapple flavor.
2.2 Administration Instructions Prior to Dosage
On the day prior to the colonoscopy, instruct patients to:
- Take only clear liquids, but avoid red and purple liquids. Patients may consume a light breakfast.
- Early in the evening prior to colonoscopy, fill the supplied container containing the GoLYTELY powder with lukewarm water (to facilitate dissolution) to the 4 liter fill line. The solution is clear and colorless when reconstituted to a final volume of 4 liters.
- After capping the container, shake vigorously several times to ensure that the ingredients are dissolved. When reconstituted use within 48 hours.
2.3 Dosage
The following is the recommended dose of reconstituted GoLYTELY solution for adults. Instruct patients they may consume water or clear liquids during the bowel preparation and after completion of the bowel preparation up until 2 hours before the time of the colonoscopy. The solution is more palatable if chilled prior to administration.
- Adults: Instruct patients to drink a total of up to 4 liters at a rate of 240 mL (8 oz.) every 10 minutes, until 4 liters are consumed or the rectal effluent is clear. Rapid drinking of each portion is preferred to drinking small amounts continuously. For NGT, rate is 20-30 mL per minute (1.2 – 1.8 liters per hour).
The first bowel movements should occur approximately one hour after the start of GoLYTELY administration. Continue drinking until the watery stool is clear and free of solid matter.
-
3 DOSAGE FORMS AND STRENGTHS
For oral solution: One 4 liter jug with powder for reconstitution with water.
Each 4 liter jug contains: polyethylene glycol 3350 236 g, sodium sulfate (anhydrous) 22.74 g, sodium bicarbonate 6.74 g, sodium chloride 5.86 g, potassium chloride 2.97 g. When made up to 4 liters volume with water, the solution contains PEG-3350 17.6 mmol/L, sodium 125 mmol/L, sulfate 40 mmol/L, chloride 35 mmol/L, bicarbonate 20 mmol/L and potassium 10 mmol/L.
-
4 CONTRAINDICATIONS
GoLYTELY is contraindicated in the following conditions:
- Gastrointestinal (GI) obstruction, ileus, or gastric retention
- Bowel perforation
- Toxic colitis or toxic megacolon
- Known allergy or hypersensitivity to any component of GoLYTELY [ see How Supplied/Storage and Handling ( 16) ]
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Serum Chemistry Abnormalities
Advise patients to hydrate adequately before, during, and after the use of GoLYTELY. Use caution in patients with congestive heart failure when replacing fluids. If a patient develops significant vomiting or signs of dehydration including signs of orthostatic hypotension after taking GoLYTELY, consider performing post-colonoscopy lab tests (electrolytes, creatinine, and BUN) and treat accordingly. Fluid and electrolyte disturbances can lead to serious adverse events including cardiac arrhythmias, seizures and renal impairment. Fluid and electrolyte abnormalities should be corrected before treatment with GoLYTELY. Advise patients to hydrate adequately before, during, and after the use of GoLYTELY. Use caution in patients with congestive heart failure when replacing fluids. If a patient develops significant vomiting or signs of dehydration including signs of orthostatic hypotension after taking GoLYTELY, consider performing post-colonoscopy lab tests (electrolytes, creatinine, and BUN) and treat accordingly. Fluid and electrolyte disturbances can lead to serious adverse events including cardiac arrhythmias, seizures and renal impairment. Fluid and electrolyte abnormalities should be corrected before treatment with GoLYTELY.
In addition, use caution when prescribing GoLYTELY for patients who have conditions, or who are using medications, that increase the risk for fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and renal impairment [ ( )] In addition, use caution when prescribing GoLYTELY for patients who have conditions, or who are using medications, that increase the risk for fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and renal impairment [ see Drug Interactions ( 7.1)]
5.2 Cardiac Arrhythmias
There have been rare reports of serious arrhythmias associated with the use of ionic osmotic laxative products for bowel preparation. Use caution when prescribing GoLYTELY for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, or cardiomyopathy). Pre-dose and post-colonoscopy ECGs should be considered in patients at increased risk of serious cardiac arrhythmias. There have been rare reports of serious arrhythmias associated with the use of ionic osmotic laxative products for bowel preparation. Use caution when prescribing GoLYTELY for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, or cardiomyopathy). Pre-dose and post-colonoscopy ECGs should be considered in patients at increased risk of serious cardiac arrhythmias.
5.3 Seizures
There have been reports of generalized tonic-clonic seizures and/or loss of consciousness associated with use of bowel preparation products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities. There have been reports of generalized tonic-clonic seizures and/or loss of consciousness associated with use of bowel preparation products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities.
Use caution when prescribing GoLYTELY for patients with a history of seizures and in patients at increased risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia. Use caution when prescribing GoLYTELY for patients with a history of seizures and in patients at increased risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia.
5.4 Renal Impairment
Use caution when prescribing GoLYTELY for patients with impaired renal function or patients taking concomitant medications that may affect renal function (such as diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or non-steroidal anti-inflammatory drugs). Advise these patients of the importance of adequate hydration, and consider performing baseline and postcolonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients. Use caution when prescribing GoLYTELY for patients with impaired renal function or patients taking concomitant medications that may affect renal function (such as diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or non-steroidal anti-inflammatory drugs). Advise these patients of the importance of adequate hydration, and consider performing baseline and postcolonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients.
5.5 Colonic Mucosal Ulcerations and Ischemic Colitis
Administration of osmotic laxative products may produce colonic mucosal aphthous ulcerations, and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of stimulant laxatives and GoLYTELY may increase this risk. The potential for mucosal ulcerations resulting from the bowel preparation should be considered when interpreting colonoscopy findings in patients with known or suspect inflammatory bowel disease (IBD). Administration of osmotic laxative products may produce colonic mucosal aphthous ulcerations, and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of stimulant laxatives and GoLYTELY may increase this risk. The potential for mucosal ulcerations resulting from the bowel preparation should be considered when interpreting colonoscopy findings in patients with known or suspect inflammatory bowel disease (IBD).
5.6 Use in Patients with Significant Gastrointestinal Disease
If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering GoLYTELY. If a patient experiences severe bloating, distention or abdominal pain, administration should be slowed or temporarily discontinued until the symptoms abate. If gastrointestinal obstruction or perforation is suspected, appropriate studies should be performed to rule out these conditions before administration of GoLYTELY. Use with caution in patients with severe active ulcerative colitis. If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering GoLYTELY. If a patient experiences severe bloating, distention or abdominal pain, administration should be slowed or temporarily discontinued until the symptoms abate. If gastrointestinal obstruction or perforation is suspected, appropriate studies should be performed to rule out these conditions before administration of GoLYTELY. Use with caution in patients with severe active ulcerative colitis.
5.7 Aspiration
Use with caution in patients with impaired gag reflex, unconscious, or semiconscious patients, and patients prone to regurgitation or aspiration. Such patients should be observed during administration of GoLYTELY, especially if it is administered via nasogastric tube. Use with caution in patients with impaired gag reflex, unconscious, or semiconscious patients, and patients prone to regurgitation or aspiration. Such patients should be observed during administration of GoLYTELY, especially if it is administered via nasogastric tube.
5.8 Not for Direct Ingestion
The contents of each jug must be diluted with water to a final volume of 4 liters (4 L) and ingestion of additional water is important to patient tolerance. Direct ingestion of the undissolved powder may increase the risk of nausea, vomiting, dehydration, and electrolyte disturbances. The contents of each jug must be diluted with water to a final volume of 4 liters (4 L) and ingestion of additional water is important to patient tolerance. Direct ingestion of the undissolved powder may increase the risk of nausea, vomiting, dehydration, and electrolyte disturbances.
-
6 ADVERSE REACTIONS
The following adverse reactions have been identified during post-approval use of GoLYTELY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nausea, abdominal fullness and bloating are the most common adverse reactions (occurred in up to 50% of patients) to administration of GoLYTELY. Abdominal cramps, vomiting and anal irritation occur less frequently. These adverse reactions are transient and usually subside rapidly. Isolated cases of urticaria, rhinorrhea, dermatitis and (rarely) anaphylactic reaction have been reported which may represent allergic reactions.
Published literature contains isolated reports of serious adverse reactions following the administration of PEG-electrolyte solution products in patients over 60 years of age. These adverse events include upper GI bleeding from Mallory-Weiss Tear, esophageal perforation, asystole, sudden dyspnea with pulmonary edema, and “butterfly-like” infiltrates on chest X-ray after vomiting and aspirating PEG.
-
7 DRUG INTERACTIONS
7.1 Drugs that May Lead to Fluid and Electrolyte Abnormalities
Use caution when prescribing GoLYTELY for patients who are using medications that increase the risk for fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and prolonged QT in the setting of fluid and electrolyte abnormalities. Consider additional patient evaluations as appropriate [ see Warnings and Precautions ( 5.1, 5.2, 5.3, and 5.4) ] in patients taking these concomitant medications.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
Animal reproduction studies have not been conducted with GoLYTELY. It is also not known whether GoLYTELY can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. GoLYTELY should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when GoLYTELY is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of GoLYTELY in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of GoLYTELY did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
11 DESCRIPTION
For oral solution: Each 4 liter (4L) GoLYTELY jug contains a white powder for reconstitution. GoLYTELY is a combination of polyethylene glycol 3350, an osmotic laxative, and electrolytes (sodium sulfate, sodium chloride, sodium bicarbonate and potassium chloride) for oral solution.
Each 4 liter jug contains: polyethylene glycol 3350 236 g, sodium sulfate (anhydrous) 22.74 g, sodium bicarbonate 6.74 g, sodium chloride 5.86 g, potassium chloride 2.97 g. The solution is clear and colorless when reconstituted to a final volume of 4 liters with water.
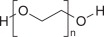
Polyethylene Glycol 3350, NF
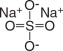
Sodium Sulfate, USP
The chemical name is Na 2SO 4. The average Molecular Weight is 142.04. The structural formula is:
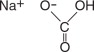
Sodium Bicarbonate, USP
The chemical name is NaHCO 3. The average Molecular Weight is 84.01. The structural formula is:
Sodium Chloride, USP
The chemical name is NaCl. The average Molecular Weight: 58.44. The structural formula is:
Na + Cl -
Potassium Chloride, USP
The chemical name is KCl. The average Molecular Weight: 74.55. The structural formula is:
K-Cl
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary mode of action is thought to be through the osmotic effect of polyethylene glycol 3350 which causes water to be retained in the colon and produces a watery stool.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
In powdered form, for oral administration as a solution following reconstitution.
GoLYTELY is available in a disposable jug and a packet in powdered form containing:
Disposable Jug: polyethylene glycol 3350 236 g, sodium sulfate (anhydrous) 22.74 g, sodium bicarbonate 6.74 g, sodium chloride 5.86 g, potassium chloride 2.97 g. When made up to 4 liters volume with water, the solution contains PEG-3350 17.6 mmol/L, sodium 125 mmol/L, sulfate 40 mmol/L, chloride 35 mmol/L, bicarbonate 20 mmol/L and potassium 10 mmol/L.
Packet: polyethylene glycol 3350 227.1 g, sodium sulfate (anhydrous) 21.5 g, sodium bicarbonate 6.36 g, sodium chloride 5.53 g, potassium chloride 2.82 g. When made up to 4 liters volume with water, the solution contains PEG-3350 60 g/L, sodium sulfate 5.86 g/L, sodium bicarbonate 1.68 g/L, sodium chloride 1.46 g/L and potassium chloride 0.745 g/L.
Pineapple Flavor GoLYTELY: polyethylene glycol 3350 236 g, sodium sulfate (anhydrous) 22.74 g, sodium bicarbonate 6.74 g, sodium chloride 5.86 g, potassium chloride 2.97 g, flavoring ingredients 3 g. When made up to 4 liters volume with water, the solution contains PEG-3350 17.6 mmol/L, sodium 125 mmol/L, sulfate 40 mmol/L, chloride 35 mmol/L, bicarbonate 20 mmol/L and potassium 10 mmol/L.
Storage:
Store in sealed container at 59° to 86°F (15°C to 30°C). When reconstituted, keep solution refrigerated. Use within 48 hours, Discard unused portion.
Keep out of reach of children.
GoLYTELY NDC: 52268-100-01
GoLYTELY Packet NDC: 52268-700-01
Pineapple Flavor GoLYTELY NDC: 52268-101-01 -
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling ( Medication Guide). Instruct patients:
- To let you know if they have trouble swallowing or are prone to regurgitation or aspiration.
- Not to take other laxatives while they are taking GoLYTELY.
- To consume water or clear liquids during the bowel preparation and after completion of the bowel preparation up until 2 hours before the time of the colonoscopy.
- That if they experience severe bloating, distention or abdominal pain, the administration of the solution should be slowed or temporarily discontinued until the symptoms abate. Advise patients to report these events to their health care provider.
- That if they have hives, rashes, or any allergic reaction, they should discontinue the medication and contact their health care provider. Medication should be discontinued until they speak to their physician.
- To contact their healthcare provider if they develop signs and symptoms of dehydration. [see Warnings and Precautions ( 5.1)].
- That oral medication administered within one hour of the start of administration of GoLYTELY solution may be flushed from the GI tract and the medication may not be absorbed completely
Distributed by Braintree Laboratories, Inc. Braintree, MA 02185
-
MEDICATION GUIDE
Medication Guide
GoLYTELY® (Go-lite-ly)
(PEG-3350 and Electrolytes for Oral Solution)
Read this Medication Guide before you start taking GoLYTELY. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about GoLYTELY?
GoLYTELY and other osmotic bowel preparations can cause serious side effects, including:
Serious loss of body fluid (dehydration) and changes in blood salts (electrolytes) in your blood.
These changes can cause:
- abnormal heartbeats that can cause death
- seizures. This can happen even if you have never had a seizure.
- kidney problems
Your chance of having fluid loss and changes in body salts with GoLYTELY is higher if you:
- have heart problems
- have kidney problems
- take water pills or non-steroidal anti-inflammatory drugs (NSAIDS)
Tell your healthcare provider right away if you have any of these symptoms of a loss of too much body fluid (dehydration) while taking GoLYTELY:
- vomiting that prevents you from keeping down the solution
- dizziness
- urinating less often than normal
- headache
See Section “ What are the possible side effects of GoLYTELY” for more information about side effects.
What is GoLYTELY?
GoLYTELY is a prescription medicine used by adults to clean the colon before a colonoscopy or barium enema X-ray examination. GoLYTELY cleans your colon by causing you to have diarrhea. Cleaning your colon helps your healthcare provider see the inside of your colon more clearly during your colonoscopy. It is not known if GoLYTELY is safe and effective in children.
Who should not take GoLYTELY?
Do not take GoLYTELY if your heathcare provider has told you that you have:
- a blockage in your bowel (obstruction)
- an opening in the wall of your stomach or intestine (bowel perforation)
- problems with food and fluid emptying from your stomach (gastric retention)
- a very dilated intestine (toxic megacolon)
- an allergy to any of the ingredients in GoLYTELY. See the end of this leaflet for a complete list of ingredients in GoLYTELY.
What should I tell my healthcare provider before taking GoLYTELY?
Before you take GoLYTELY, tell your healthcare provider if you:
- have heart problems
- have stomach or bowel problems
- have ulcerative colitis
- have problems with swallowing or gastric reflux
- have a history of seizures
- are withdrawing from drinking alcohol
- have a low blood salt (sodium) level
- have kidney problems
- any other medical conditions
- are pregnant. It is not known if GoLYTELY will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if GoLYTELY passes into your breast milk. You and your healthcare provider should decide if you will take GoLYTELY while breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. GoLYTELY may affect how other medicines work. Medicines taken by mouth may not be absorbed properly when taken within 1 hour before the start of GoLYTELY.
Especially tell your healthcare provider if you take:
- medicines for blood pressure or heart problems
- medicines for kidney problems
- medicines for seizures
- water pills (diuretics)
- non-steroidal anti-inflammatory medicines (NSAID) pain medicines
- laxatives
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure if you are taking any of the medicines listed above.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take GoLYTELY?
You must read, understand, and follow these instructions to take GoLYTELY the right way.
- Take GoLYTELY exactly as your healthcare provider tells you to take it.
- Drink 240 mL (8 oz.) every 10 minutes. Rapid drinking of each portion is better than drinking small amounts.
- The first bowel movement should occur approximately one hour after you start drinking the solution.
- You may experience some abdominal bloating and distention before the bowels start to move. If severe discomfort or distention occur, stop drinking temporarily or drink each portion at longer intervals until the discomfort goes away.
- Continue drinking until the watery stool is clear and free of solid matter. This usually requires 3 liters and it is best to drink all of the solution.
- Do not take undissolved GoLYTELY powder that has not been mixed with water (diluted), it may increase your risk of nausea, vomiting and fluid loss (dehydration).
- Each jug of GoLYTELY must be reconstituted with water (diluted) to 4 liters total volume before drinking.
- Do not take other laxatives while taking GoLYTELY.
-
Do not eat solid foods on the day before your colonoscopy and until after your colonoscopy. Drink only clear liquids:
- the day before your colonoscopy
- while taking GoLYTELY
- after taking GoLYTELY until 2 hours before your colonoscopy
Do not eat or drink anything 2 hours before your colonoscopy.
- Drink clear liquids before, during, and after you take GoLYTELY to avoid fluid loss (dehydrated). Examples of clear liquids are:
- water
- clear fruit juices without pulp including apple, white grape, or white cranberry
- strained limeade or lemonade
- coffee or tea (Do not use any dairy or non-dairy creamer)
- clear broth
- clear soda
- gelatin (without added fruit or topping)
- popsicles without pieces of fruit or fruit pulp
Do not eat or drink anything colored red or purple.
What are the possible side effects of GoLYTELY?
GoLYTELY can cause serious side effects, including:
- See Section “ What is the most important information I should know about GoLYTELY?”
-
changes in certain blood tests. Your healthcare provider may do blood tests after you take GoLYTELY to check your blood for changes. Tell your healthcare provider if you have any symptoms of too much fluid loss, including:
- vomiting
- nausea
- bloating
- dizziness
- stomach (abdominal) cramping
- headache
- urinate less than usual
- trouble drinking clear liquid
- heart problems. GoLYTELY may cause irregular heartbeats.
- seizures
- ulcers of the bowel or bowel problems (ischemic colitis). Tell your healthcare provider right away if you have severe stomach-area (abdomen) pain or rectal bleeding.
The most common side effects of GoLYTELY include:
- nausea
- stomach (abdominal) fullness
- bloating
- stomach (abdominal) cramps
- vomiting
- anal irritation
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of GoLYTELY. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store GoLYTELY?
-
Store GoLYTELY at room temperature, between 59°F to 86°F (15°C to 30°C).
Keep GoLYTELY and all medicines out of the reach of children.
General information about the safe and effective use of GoLYTELY.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use GoLYTELY for a condition for which it was not prescribed. Do not give GoLYTELY to other people, even if they are going to have the same procedure you are. It may harm them.
This Medication Guide summarizes important information about GoLYTELY. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information that is written for healthcare professionals.
For more information, go to www.braintreelabs.com or call 1-800-874-6756.
What are the ingredients in GoLYTELY?
Active ingredients: polyethylene glycol 3350, sodium sulfate, sodium bicarbonate, sodium chloride, and potassium chloride.
Inactive ingredients: Pineapple Flavored GoLYTELY only (natural and artificial pineapple flavor powder, maltodextrin, gum arabic, sodium saccharin, silicon dioxide)
Braintree Laboratories, Inc.
Braintree, MA 02185, USA
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised 9/2013
-
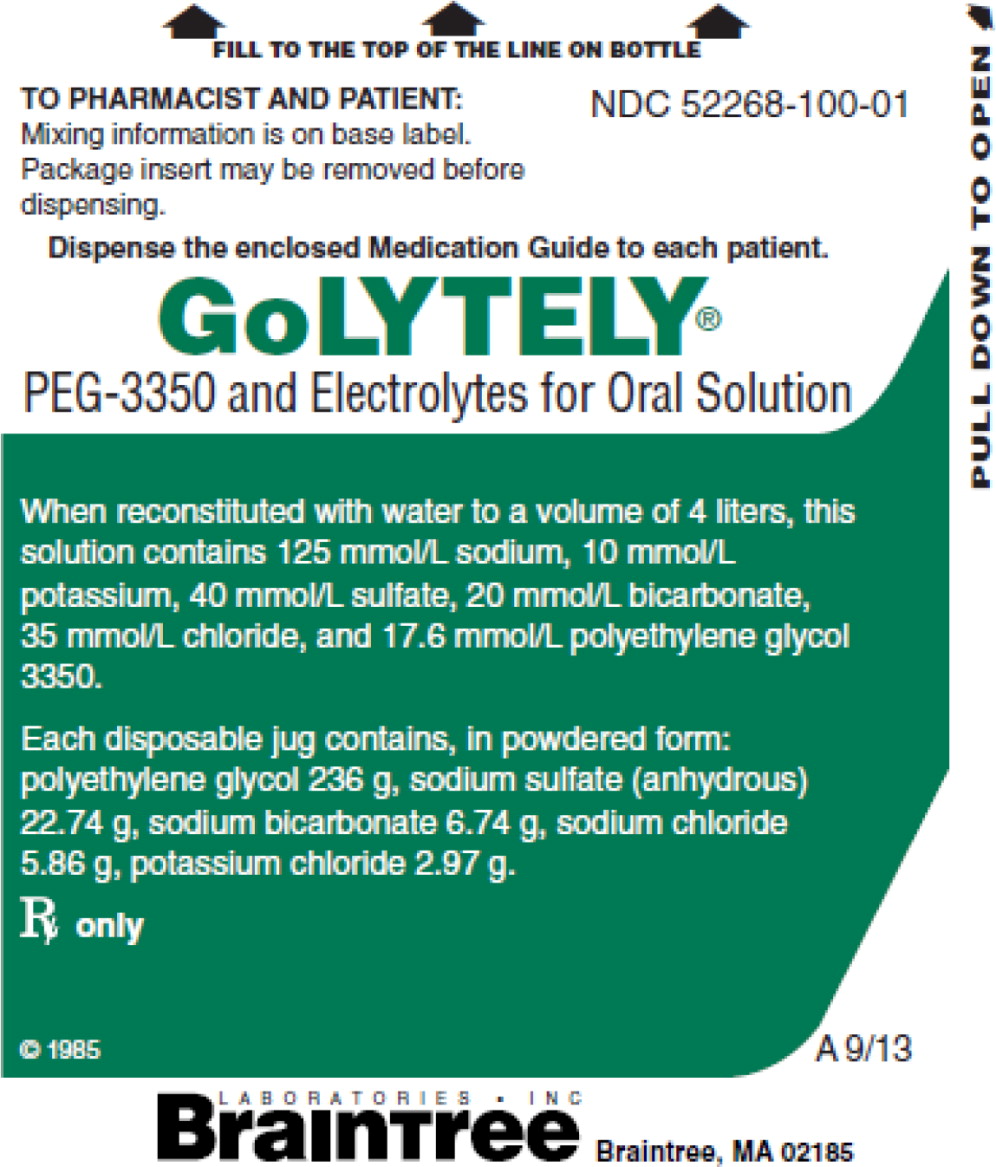
PRINCIPAL DISPLAY PANEL
Principal Display Panel
FILL TO THE TOP OF THE LINE ON BOTTLE
NDC: 52268-100-01
TO PHARMACIST AND PATIENT:
Mixing information is on base
label. Package insert may be
removed before dispensing.Dispense the enclosed Medication Guide to each patient.
GoLYTELY®
PEG-3350 and Electrolytes for Oral Solution
When reconstituted with water to a volume of 4 liters, this solution contains 125 mmol/L sodium , 10 mmol/L potassium, 40 mmol/L sulfate, 20 mmol/L bicarbonate, 35 mmol/L chloride and 17.6 mmol/L polyethylene glycol 3350.
Each disposable jug contains, in powdered form: polyethylene glycol 3350 236 g, sodium sulfate (anhydrous) 22.74 g, sodium bicarbonate 6.74 g, sodium chloride 5.86 g, potassium chloride 2.97 g.
RX only M 9/13
Braintree
Laboratories, Inc
Braintree, MA 02185
-
PRINCIPAL DISPLAY PANEL
FILL TO THE TOP OF THE LINE ON BOTTLE
NDC: 52268-101-01
TO PHARMACIST AND PATIENT:
Mixing information is on base
label. Package insert may be
removed before dispensing. Dispense the enclosed Medication Guide to each patient.Pineapple Flavor
GoLYTELY®
PEG-3350 and Electrolytes for Oral Solution
When reconstituted with water to a volume of 4 liters, this solution contains 125 mmol/L sodium , 10 mmol/L potassium, 40 mmol/L sulfate, 20 mmol/L bicarbonate, 35 mmol/L chloride and 17.6 mmol/L polyethylene glycol 3350.
Each disposable jug contains, in powdered form: polyethylene glycol 3350 236 g, sodium sulfate (anhydrous) 22.74 g, sodium bicarbonate 6.74 g, sodium chloride 5.86 g, potassium chloride 2.97 g, flavor ingredients 3.0 g.
RX only M 9/13
Braintree
Laboratories, Inc
Braintree, MA 02185
-
PRINCIPAL DISPLAY PANEL
NDC: 52268-700-01
Rx onlyGoLYTELY®
PEG-3350 AND ELECTROLYTES
FOR ORAL SOLUTION
1 Gallon (3.785 Liters)Instructions
A GoLYTELY® solution is made up by dissolving the contents of this packet in one gallon of tap
water according to the following procedure:- Obtain a food-grade clear container with a volume of at least one gallon.
- After cutting open the packet, pour the entire contents into the container.
- Add lukewarm drinking water to bring the volume of the solution to one gallon.
- Do not add any other ingredients, flavors, etc.
- Shake and/or mix thoroughly to ensure that the ingredients are dissolved.
- The solution is more palatable if chilled in the refrigerator before drinking. Keep reconstituted solution refrigerated. Use within 48 hours. Discard unused portion.
- For best results, no solid food should be consumed for the 3 to 4 hour period before drinking the solution; but in no case should solid food be eaten within two hours of taking GoLYTELY.
- Drink one 8 oz. glassful of the solution rapidly every 10 minutes. A loose watery bowel movement should result in approximately one hour. Continue drinking until the rectal effluent is clear or the entire contents (1 gallon) have been consumed or as directed by your physician.
Usual dosage: 1 gallon (3.785 liters).
This package contains:
Polyethylene glycol 3350…..227.1 grams
Sodium sulfate, anhydrous….21.5 grams
Sodium bicarbonate………….6.36 grams
Sodium chloride………………5.53 grams
Potassium chloride…………..2.82 gramsAPPROXIMATE NET WEIGHT: 263 grams
Distributed by Braintree Laboratories, Inc., Braintree, MA 02185 -
INGREDIENTS AND APPEARANCE
GOLYTELY
polyethylene glycol 3350, sodium sulfate anhydrous, sodium bicarbonate, sodium chloride, potassium chloride powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52268-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 236 g in 4 L SODIUM SULFATE ANHYDROUS (UNII: 36KCS0R750) (SODIUM CATION - UNII:LYR4M0NH37, SULFATE ION - UNII:7IS9N8KPMG) SODIUM SULFATE ANHYDROUS 22.74 g in 4 L SODIUM BICARBONATE (UNII: 8MDF5V39QO) (SODIUM CATION - UNII:LYR4M0NH37, BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 6.74 g in 4 L SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 5.86 g in 4 L POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 2.97 g in 4 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52268-100-01 4 L in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/13/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019011 07/13/1984 GOLYTELY
polyethylene glycol 3350, sodium sulfate anhydrous, sodium bicarbonate, sodium chloride, potassium chloride powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52268-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 236 g in 4 L SODIUM SULFATE ANHYDROUS (UNII: 36KCS0R750) (SODIUM CATION - UNII:LYR4M0NH37, SULFATE ION - UNII:7IS9N8KPMG) SODIUM SULFATE ANHYDROUS 22.74 g in 4 L SODIUM BICARBONATE (UNII: 8MDF5V39QO) (SODIUM CATION - UNII:LYR4M0NH37, BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 6.74 g in 4 L SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 5.86 g in 4 L POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 2.97 g in 4 L Product Characteristics Color Score Shape Size Flavor PINEAPPLE (PINEAPPLE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52268-101-01 4 L in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/13/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019011 07/13/1984 GOLYTELY
polyethylene glycol 3350, sodium sulfate anhydrous, sodium bicarbonate, sodium chloride, potassium chloride powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52268-700 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 227.1 g in 1 L SODIUM SULFATE ANHYDROUS (UNII: 36KCS0R750) (SODIUM CATION - UNII:LYR4M0NH37, SULFATE ION - UNII:7IS9N8KPMG) SODIUM SULFATE ANHYDROUS 21.5 g in 1 L SODIUM BICARBONATE (UNII: 8MDF5V39QO) (SODIUM CATION - UNII:LYR4M0NH37, BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 6.36 g in 1 L SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 5.53 g in 1 L POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 0.754 g in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52268-700-01 4 L in 1 PACKET; Type 0: Not a Combination Product 07/13/1984 05/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019011 07/13/1984 05/31/2022 Labeler - Braintree Laboratories, Inc. (107904591) Establishment Name Address ID/FEI Business Operations Braintree Laboratories, Inc. 617357954 manufacture(52268-100, 52268-101, 52268-700)
Trademark Results [GoLYTELY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GOLYTELY 73409969 1386512 Live/Registered |
BRAINTREE LABORATORIES, INC. 1983-01-19 |
 GOLYTELY 73266701 not registered Dead/Abandoned |
BAYLOR UNIVERSITY MEDICAL CENTER 1980-06-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.