VANCOMYCIN injection, solution
Vancomycin by
Drug Labeling and Warnings
Vancomycin by is a Prescription medication manufactured, distributed, or labeled by Xellia Pharmaceuticals USA LLC, Xellia Pharmaceuticals ApS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VANCOMYCIN INJECTION safely and effectively. See full prescribing information for VANCOMYCIN INJECTION.
VANCOMYCIN injection, for intravenous use
Initial U.S. Approval: 1958WARNING: RISK OF EMBRYO-FETAL TOXICITY DUE TO EXCIPIENTS
See full prescribing information for complete boxed warning.
This formulation of Vancomycin Injection is not recommended for use during pregnancy because it contains the excipients polyethylene glycol (PEG 400) and N-acetyl D-alanine (NADA), which caused fetal malformations in animal reproduction studies. If use of vancomycin is needed during pregnancy, use other available formulations of vancomycin. (5.1, 8.1)
INDICATIONS AND USAGE
Vancomycin Injection is a glycopeptide antibacterial indicated in adult and pediatric patients (1 month and older) for the treatment of:
- Septicemia (1.1)
- Infective Endocarditis (1.2)
- Skin and Skin Structure Infections (1.3)
- Bone Infections (1.4)
- Lower Respiratory Tract Infections (1.5)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Injection and other antibacterial drugs, Vancomycin Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. (1.6)
DOSAGE AND ADMINISTRATION
- Use this formulation of Vancomycin Injection only in patients who require the entire (500 mg, 1 g, 1.5 g or 2 g) dose and not any fraction thereof. (2.1)
- For intravenous use only. Do Not administer orally.
- Administer Vancomycin Injection by intravenous infusion over 60 minutes or greater to reduce the risk of infusion reactions (2.1)
- Adult Patients: 2 g divided either as 0.5 grams (g) every 6 hours or 1 g every 12 hours (2.2)
- Pediatric Patients (1 Month and Older): 10 mg/kg per dose given every 6 hours (2.3)
- Patients with Renal Impairment: See full prescribing information for recommended doses in patients with renal impairment (2.4)
- See full prescribing information for further important administration and preparation instructions (2.1, 2.5)
DOSAGE FORMS AND STRENGTHS
Vancomycin Injection: Single-dose flexible bags containing 500 mg vancomycin in 100 mL, 1 g vancomycin in 200 mL, 1.5 g vancomycin in 300 mL and 2 g vancomycin in 400 mL of liquid (3).
CONTRAINDICATIONS
Hypersensitivity to vancomycin (4)
WARNINGS AND PRECAUTIONS
- Infusion Reactions: Hypotension, including shock and cardiac arrest, wheezing, dyspnea, urticaria, muscular and chest pain and "red man syndrome" which manifests as pruritus and erythema that involves the face, neck and upper torso may occur with rapid intravenous administration. To reduce the risk of infusion reactions, administer Vancomycin Injection over a period of 60 minutes or greater and also prior to intravenous anesthetic agents. (2.1, 5.2)
- Nephrotoxicity: Systemic vancomycin exposure may result in acute kidney injury (AKI) including acute renal failure, mainly due to interstitial nephritis or less commonly acute tubular necrosis. Monitor serum vancomycin concentrations and renal function. (5.3)
- Ototoxicity: Ototoxicity has occurred in patients receiving vancomycin. Monitor for signs and symptoms of ototoxicity during therapy. Monitor serum vancomycin concentrations and renal function. Assessment of auditory function may be appropriate in some instances. (5.4)
- Clostridium Difficile-Associated Diarrhea: Evaluate patients if diarrhea occurs. (5.5).
- Neutropenia: Periodically monitor leukocyte count. (5.7)
- Phlebitis: To reduce the risk of local irritation and phlebitis administer Vancomycin Injection by a secure intravenous route of administration. (5.8)
- Development of Drug-Resistant Bacteria: Prescribing Vancomycin Injection in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug resistant bacteria. (5.9)
ADVERSE REACTIONS
The common adverse reactions are anaphylaxis, "red man syndrome", acute kidney injury, hearing loss, neutropenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Xellia Pharmaceuticals USA, LLC at 1-833-295-6953 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Anesthetic Agents: Concomitant administration of vancomycin and anesthetic agents has been associated with erythema and histamine-like flushing. (2.1, 7.1)
- Piperacillin/Tazobactam: Increased incidence of acute kidney injury in patients receiving concomitant piperacillin/tazobactam and vancomycin as compared to vancomycin alone. Monitor kidney function in patients (7.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING:RISK OF EMBRYO-FETAL TOXICITY DUE TO EXCIPIENTS
1 INDICATIONS AND USAGE
1.1 Septicemia
1.2 Infective Endocarditis
1.3 Skin and Skin Structure Infections
1.4 Bone Infections
1.5 Lower Respiratory Tract Infections
1.6 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage in Adult Patients with Normal Renal Function
2.3 Dosage in Pediatric Patients (1 Month and Older) with Normal Renal Function
2.4 Dosage in Patients with Renal Impairment
2.5 Directions for Use of Vancomycin Injection and Storage Instructions
2.6 Incompatibilities for Intravenous Use
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Embryo-Fetal Toxicity Due to PEG 400 and NADA Excipients
5.2 Infusion Reactions
5.3 Nephrotoxicity
5.4 Ototoxicity
5.5 Clostridium Difficile-Associated Diarrhea (CDAD)
5.6 Hemorrhagic Occlusive Retinal Vasculitis (HORV)
5.7 Neutropenia
5.8 Phlebitis and Other Administration Site Reactions
5.9 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Anesthetic Agents
7.2 Piperacillin-Tazobactam
7.3 Ototoxic and/or Nephrotoxic Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING:RISK OF EMBRYO-FETAL TOXICITY DUE TO EXCIPIENTS
This formulation of Vancomycin Injection is not recommended for use during pregnancy because it contains the excipients polyethylene glycol (PEG) 400 and N-acetyl-D-alanine (NADA), which caused fetal malformations in animal reproduction studies. If use of vancomycin is needed during pregnancy, use other available formulations of vancomycin [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
-
1 INDICATIONS AND USAGE
1.1 Septicemia
Vancomycin Injection is indicated in adults and pediatric patients (1 month and older) for the treatment of septicemia due to:
- Susceptible isolates of methicillin-resistant Staphylococcus aureus (MRSA) and coagulase negative staphylococci.
- Methicillin-susceptible staphylococci in penicillin-allergic patients, or those patients who cannot receive or who have failed to respond to other drugs, including penicillins or cephalosporins.
1.2 Infective Endocarditis
Vancomycin Injection is indicated in adults and pediatric patients (1 month and older) for the treatment of infective endocarditis due to:
- Susceptible isolates of MRSA.
- Viridans group streptococci Streptococcus gallolyticus (previously known as Streptococcus bovis), Enterococcus species and Corynebacterium species. For enterococcal endocarditis, use Vancomycin Injection in combination with an aminoglycoside.
- Methicillin-susceptible staphylococci in penicillin-allergic patients, or those patients who cannot receive or who have failed to respond to other drugs, including penicillins or cephalosporins.
Vancomycin Injection is indicated in adults and pediatric patients (1 month and older) for the treatment of early-onset prosthetic valve endocarditis caused by Staphylococcus epidermidis in combination with rifampin and an aminoglycoside.
1.3 Skin and Skin Structure Infections
Vancomycin Injection is indicated in adults and pediatric patients (1 month and older) for the treatment of skin and skin structure infections due to:
- Susceptible isolates of MRSA and coagulase negative staphylococci.
- Methicillin-susceptible staphylococci in penicillin-allergic patients, or those patients who cannot receive or who have failed to respond to other drugs, including penicillins or cephalosporins.
1.4 Bone Infections
Vancomycin Injection is indicated in adults and pediatric patients (1 month and older) for the treatment of bone infections due to:
- Susceptible isolates of MRSA and coagulase negative staphylococci.
- Methicillin-susceptible staphylococci in penicillin-allergic patients, or those patients who cannot receive or who have failed to respond to other drugs, including penicillins or cephalosporins.
1.5 Lower Respiratory Tract Infections
Vancomycin Injection is indicated in adults and pediatric patients (1 month and older) for the treatment of lower respiratory tract infections due to:
- Susceptible isolates of MRSA
- Methicillin-susceptible staphylococci in penicillin-allergic patients, or those patients who cannot receive or who have failed to respond to other drugs, including penicillins or cephalosporins.
1.6 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Injection and other antibacterial drugs, Vancomycin Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Use this formulation of Vancomycin Injection only in patients who require the entire (500 mg, 1 g, 1.5 g or 2 g) dose and not any fraction thereof.
Vancomycin Injection in transparent single-dose flexible bags are intended for intravenous use only. Do NOT administer orally.
To reduce the risk of infusion related adverse reactions, administer Vancomycin Injection by intravenous infusion over 60 minutes or greater [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)]. An infusion rate of 10 mg/min or less is associated with fewer infusion-related events [see Warnings and Precautions (5.2)]. Infusion related events may occur, however, at any rate or concentration.
Drug additives should not be made to this solution.
Vancomycin Injection concentrations of no more than 5 mg/mL are recommended in adults [see Dosage and Administration (2.2)]. See also age-specific recommendations [see Dosage and Administration (2.3)].
Administer Vancomycin Injection prior to intravenous anesthetic agents to reduce the risk of infusion related adverse reactions [see Warnings and Precautions (5.2)].
Administer Vancomycin Injection by a secure intravenous route of administration to avoid local irritation and phlebitis reactions [see Warnings and Precautions (5.8)].
2.2 Dosage in Adult Patients with Normal Renal Function
The usual daily intravenous dose is 2 g divided either as 500 mg every 6 hours or 1 g every 12 hours. Administer each dose by intravenous infusion over a period of 60 minutes or greater. Other patient factors, such as age or obesity, may call for modification of the usual intravenous daily dose. The initial daily dose should be no less than 15 mg/kg.
2.3 Dosage in Pediatric Patients (1 Month and Older) with Normal Renal Function
Use this formulation of Vancomycin Injection only in pediatric patients (1 month and older) who require the entire dose (500 mg, 1 g, 1.5 g or 2 g) of this single-dose flexible bag and not any fraction of it [see Dosage Forms and Strengths (3)].
The usual intravenous dosage of vancomycin is 10 mg/kg per dose given every 6 hours. Each dose should be administered over a period of at least 60 minutes. Close monitoring of serum concentrations of vancomycin may be warranted in these patients.
2.4 Dosage in Patients with Renal Impairment
Dosage adjustment must be made in patients with renal impairment. The initial dose should be no less than 15 mg/kg in patients with any degree of renal impairment.
In the elderly, greater dosage reductions than expected may be necessary because of decreased renal function. Measure trough vancomycin serum concentrations to guide therapy, especially in seriously ill patients with changing renal function.
For functionally anephric patients, an initial dose of 15 mg/kg of body weight should be given to achieve prompt therapeutic serum concentration. A dose of 1.9 mg/kg/24 h should be given after the initial dose of 15 mg/kg.
2.5 Directions for Use of Vancomycin Injection and Storage Instructions
Vancomycin Injection, in transparent single-dose flexible bag is for intravenous administration only.
Vancomycin Injection is room temperature stable, ready-to-use drug product.
Preparation for Intravenous Administration:
- Remove the flexible bag from aluminum overpouch.
- Check for minute leaks by squeezing the bag firmly. If leaks are detected, discard solution because sterility may be impaired. Leaks may be more readily detected by wrapping the bag with blotting paper or a tissue before squeezing.
- Do not add supplemental medication.
- Visually inspect the flexible bag. If the outlet port protector is damaged, detached, or not present, discard the flexible bag as solution path sterility may be impaired. If after visual inspection the solution is cloudy or if an insoluble precipitate is noted or if any seals are not intact, the flexible bag should be discarded.
- The solution in the flexible bag remains chemically stable for 28 days at room temperature (up to 25°C/77°F) after removal from the aluminum overpouch. Discard unused drug.
- Suspend the flexible bag from eyelet support.
- Remove protector from outlet port at bottom of flexible bag.
- Attach administration set. Refer to complete directions accompanying set.
- Use sterile equipment.
Do NOT use flexible bags in series connections. Such use could result in an embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete.
2.6 Incompatibilities for Intravenous Use
Vancomycin solution has a low pH and may cause chemical or physical instability when it is mixed with other compounds.
Mixtures of solutions of vancomycin and beta-lactam antibacterial drugs have been shown to be physically incompatible. The likelihood of precipitation increases with higher concentrations of vancomycin. It is recommended to adequately flush the intravenous lines between the administration of these antibacterial drugs.
-
3 DOSAGE FORMS AND STRENGTHS
Vancomycin Injection is a ready to use clear, colorless to light brown solution in single-dose flexible bags containing 500 mg vancomycin in 100 mL, 1 g vancomycin in 200 mL, 1.5 g vancomycin in 300 mL, and 2 g vancomycin in 400 mL of liquid [see Description (11)]. The flexible bags are supplied in sealed aluminum overpouches.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Embryo-Fetal Toxicity Due to PEG 400 and NADA Excipients
This formulation of Vancomycin Injection is not recommended during pregnancy because it contains the excipients PEG 400 and NADA, which caused fetal malformations in animal reproduction studies. The active ingredient vancomycin is not known to be associated with embryo-fetal toxicity [see BOXED WARNING, Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Advise pregnant women of the potential risk to the fetus. If use of vancomycin is needed during pregnancy, use other available formulations of vancomycin.
5.2 Infusion Reactions
Hypotension, including shock and cardiac arrest, wheezing, dyspnea, urticaria, muscular and chest pain may occur with rapid Vancomycin Injection administration. The reactions may be more severe in younger patients, particularly children, and in patients receiving concomitant muscle relaxant anesthetics.
Rapid intravenous administration of Vancomycin Injection may also be associated with "red man syndrome", which manifests as pruritus and erythema that involves the face, neck and upper torso.
Infusion-related adverse reactions are related to both the concentration and the rate of administration of vancomycin. Infusion-related adverse reactions may occur, however, at any rate or concentration.
Administer Vancomycin Injection over a period of 60 minutes or greater to reduce the risk of infusion-related adverse reactions. In selected patients in need of fluid restriction, a concentration up to 10 mg/mL may be used; use of such higher concentrations may increase the risk of infusion-related adverse reactions. Administer prior to intravenous anesthetic agents when feasible. Stop the infusion if a reaction occurs.
5.3 Nephrotoxicity
Vancomycin Injection can result in acute kidney injury (AKI), including acute renal failure, mainly due to interstitial nephritis or less commonly acute tubular necrosis. AKI is manifested by increasing blood urea nitrogen (BUN) and serum creatinine (Cr). The risk of AKI increases with higher vancomycin serum levels, prolonged exposure, concomitant administration of other nephrotoxic drugs, concomitant administration of piperacillin-tazobactam [see Drug Interactions (7.2)], volume depletion, pre-existing renal impairment and in critically ill patients and patients with co-morbid conditions that predispose to renal impairment.
Monitor serum vancomycin concentrations and renal function in all patients receiving Vancomycin Injection. More frequent monitoring is recommended in patients with comorbidities that predispose to impairment in renal function or are concomitantly receiving other nephrotoxic drugs, in critically ill patients, in patients with changing renal function, and in patients requiring higher therapeutic vancomycin levels. If acute kidney injury occurs, discontinue Vancomycin Injection or reduce the dose.
5.4 Ototoxicity
Ototoxicity has occurred in patients receiving vancomycin. It may be transient or permanent. Ototoxicity manifests as tinnitus, hearing loss, dizziness or vertigo. The risk is higher in older patients, patients who are receiving higher doses, who have an underlying hearing loss, who are receiving concomitant therapy with another ototoxic agent, such as an aminoglycoside or who have underlying renal impairment. Monitor for signs and symptoms of ototoxicity during therapy. Monitor serum vancomycin concentrations and renal function in all patients receiving parenteral vancomycin. Discontinue Vancomycin Injection if ototoxicity occurs. Dosage of Vancomycin Injection must be adjusted for patients with renal impairment [see Dosage and Administration (2.3)]. Serial tests of auditory function may be helpful in order to minimize the risk of ototoxicity.
5.5 Clostridium Difficile-Associated Diarrhea (CDAD)
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including vancomycin and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Clinically significant serum concentrations have been reported in some patients being treated for active C. difficile-induced pseudomembranous colitis after multiple oral doses of vancomycin.
Prolonged use of Vancomycin Injection may result in the overgrowth of nonsusceptible microorganisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken. In rare instances, there have been reports of pseudomembranous colitis due to C. difficile developing in patients who received intravenous vancomycin.
5.6 Hemorrhagic Occlusive Retinal Vasculitis (HORV)
Hemorrhagic occlusive retinal vasculitis, including permanent loss of vision, occurred in patients receiving intracameral or intravitreal administration of vancomycin during or after cataract surgery. The safety and efficacy of vancomycin administered by the intracameral or the intravitreal route have not been established by adequate and well-controlled trials. Vancomycin is not indicated for the prophylaxis of endophthalmitis.
5.7 Neutropenia
Reversible neutropenia has been reported in patients receiving vancomycin [see Adverse Reactions (6.1)]. Patients who will undergo prolonged therapy with vancomycin or those who are receiving concomitant drugs which may cause neutropenia should have periodic monitoring of the leukocyte count.
5.8 Phlebitis and Other Administration Site Reactions
Inflammation at the site of injection of vancomycin has been reported. Vancomycin is irritating to tissue and must be given by a secure intravenous route of administration to reduce the risk of local irritation and phlebitis.
Administration of vancomycin by intramuscular (IM), intraperitoneal, intrathecal (intralumbar or intraventricular), or intravitreal routes has not been approved and is not recommended. The safety and efficacy of vancomycin administered by the intrathecal (intralumbar or intraventricular) route or by the intraperitoneal route have not been established by adequate and well controlled trials.
Pain, tenderness, and necrosis occur with IM injection of vancomycin or with inadvertent extravasation. Thrombophlebitis may occur, the frequency and severity of which can be minimized by slow infusion of the drug and by rotation of venous access sites.
Intraperitoneal administration during continuous ambulatory peritoneal dialysis (CAPD) can result in chemical peritonitis. Manifestations range from cloudy dialysate alone to a cloudy dialysate accompanied by variable degrees of abdominal pain and fever. This syndrome appears to be resolved after discontinuation of intraperitoneal vancomycin.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Infusion Reactions [see Warnings and Precautions (5.2)]
- Nephrotoxicity [see Warnings and Precautions (5.3)]
- Ototoxicity [see Warnings and Precautions (5.4)]
- Clostridium Difficile-Associated Diarrhea [see Warnings and Precautions (5.5)]
- Hemorrhagic Occlusive Retinal Vasculitis [see Warnings and Precautions (5.6)]
- Neutropenia [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reactions associated with the use of vancomycin were identified in clinical trials:
Immune system disorders: Hypersensitivity reactions including anaphylaxis and "red man syndrome" [see Warnings and Precautions (5.2)]
Skin and subcutaneous tissue disorders: Erythema (especially of the face, neck and upper torso) and pruritus which are manifestations of rashes including exfoliative dermatitis, linear IgA bullous dermatosis, Stevens-Johnson syndrome, toxic epidermal necrolysis
Renal and urinary disorders: Acute kidney injury and interstitial nephritis
Ear and Labyrinth Disorders: Tinnitus, hearing loss, vertigo
Blood and Lymphatic System Disorders: Agranulocytosis, neutropenia, pancytopenia, leukopenia, thrombocytopenia, eosinophilia
Gastrointestinal Disorders: Pseudomembranous colitis [see Warnings and Precautions (5.5)]
Cardiac Disorders: Cardiac arrest, chest pain
General Disorders and Administration Site Conditions: General discomfort, fever, chills, phlebitis, injection site irritation, injection site pain and necrosis following intramuscular injection, chemical peritonitis following intraperitoneal administration (Vancomycin Injection is not approved for intramuscular and intraperitoneal administration) [see Warnings and Precautions (5.8)]
Laboratory Abnormalities: Elevated blood urea nitrogen, elevated serum creatinine
Musculoskeletal and connective tissue disorders: Muscle pain
Nervous system disorders: Dizziness
Respiratory, thoracic and mediastinal disorders: Wheezing, dyspnea
Vascular disorders: Hypotension, shock, vasculitis
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of vancomycin. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders: Drug Rash with Eosinophilia and Systemic Symptoms (DRESS)
-
7 DRUG INTERACTIONS
7.1 Anesthetic Agents
Concomitant administration of vancomycin and anesthetic agents has been associated with erythema and histamine-like flushing [see Warnings and Precautions (5.2) and Use in Specific Populations (8.4)].
7.2 Piperacillin-Tazobactam
Studies have detected an increased incidence of acute kidney injury in patients administered concomitant piperacillin/tazobactam and vancomycin as compared to vancomycin alone. Monitor kidney function in patients receiving concomitant piperacillin/tazobactam and vancomycin. No pharmacokinetic interactions have been noted between piperacillin/tazobactam and vancomycin.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
This formulation of Vancomycin Injection is not recommended for use during pregnancy because it contains the excipients, PEG 400 and NADA, which caused fetal malformations in animal reproduction studies (see Data). Advise pregnant women of the potential risk to the fetus. If therapy with vancomycin is needed during pregnancy, use other available formulations of vancomycin.
There are no available data on vancomycin use in pregnant women to inform a drug-associated risk of major birth defects or miscarriage. Available published data on vancomycin use in pregnancy during the second and third trimesters have not shown an association with adverse pregnancy related outcomes (see Data). There are no available data on first trimester use of vancomycin, including vancomycin with the excipients PEG 400 and NADA, in pregnant women to assess the risk of major birth defects or miscarriage. Vancomycin alone did not show adverse developmental effects when administered intravenously to pregnant rats and rabbits during organogenesis at doses less than or equal to the recommended maximum human dose based on body surface area.
Reproduction studies in rabbits with intravenous doses of PEG 400 at approximately 5 times the maximum daily human dose based on body surface area comparisons administered during organogenesis resulted in fetal spinal malformations. Reproduction studies in rabbits and rats using intravenous doses of NADA at approximately 6 and 7 times the maximum daily human dose, respectively, based on body surface area comparisons resulted in maternal toxicity and fetal spinal and cardiovascular malformations in rabbits, and maternal toxicity with no significant adverse embryo-fetal effects in rats. Vancomycin alone did not show adverse developmental effects when administered intravenously to pregnant rats and rabbits during organogenesis at doses less than or equal to the recommended maximum human dose based on body surface area (see Data).
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
There are no available data on first trimester use of vancomycin, including vancomycin with the excipients PEG 400 and NADA, in pregnant women to assess a risk of major birth defects or miscarriage.
A published study evaluated hearing loss and nephrotoxicity in infants of 10 pregnant intravenous drug users treated with vancomycin (formulation did not include the excipients PEG 400 and NADA) for suspected or documented methicillin-resistant Staphylococcus aureus in the second or third trimester.
The comparison groups were 10 uninfected non-intravenous drug-dependent patients, and 10 uninfected intravenous drug-dependent patients who served as substance abuse controls. No infant in the vancomycin exposed group had abnormal sensorineural hearing at 3 months of age or nephrotoxicity.
A published prospective study assessed outcomes in 55 pregnant women with a positive Group B streptococcus (GBS) culture and a high-risk penicillin allergy with resistance to clindamycin or unknown sensitivity who were administered vancomycin (formulation did not include the excipients PEG 400 and NADA) at the time of delivery. Vancomycin dosing ranged from the standard 1 g intravenously every 12 hours to 20 mg/kg intravenous every 8 hours (maximum individual dose 2 g). No major adverse reactions were recorded either in the mothers or their newborns. None of the newborns had sensorineural hearing loss. Neonatal renal function was not examined, but all of the newborns were discharged in good condition.
Animal Data
Vancomycin did not cause fetal malformations when administered during organogenesis to pregnant rats (gestation days 6 to 15) and rabbits (gestation days 6 to 18) at the equivalent recommended maximum human dose (based on body surface area comparisons) of 200 mg/kg/day IV to rats or 120 mg/kg/day IV to rabbits. No effects on fetal weight or development were seen in rats at the highest dose tested or in rabbits given 80 mg/kg/day (approximately 1 and 0.8 times the recommended maximum human dose based on body surface area, respectively). Maternal toxicity was observed in rats (at doses 120 mg/kg and above) and rabbits (at 80 mg/kg and above).
Animal reproduction studies conducted in rabbits administered intravenous PEG 400 at 2000 mg/kg (approximately 5 times the maximum daily human dose, based on body surface comparisons) during organogenesis (gestation days 6 to 19) resulted in fetal scoliosis (thoracic and lumbar) and increased incidence of delayed or incomplete ossification of the pubes, epiphyses, and talus bones. No maternal toxicity was observed up to the maximum dose tested.
Similarly, in animal reproduction studies conducted in pregnant rabbits (gestation days 6 to19) and pregnant rats (gestation days 6 to 17) administered intravenous NADA at 1680 and 3780 mg/kg, respectively (approximately 6 and 7 times the maximum daily human dose, respectively, based on body surface comparisons) resulted in fetal scoliosis and a spectrum of rare cardiovascular anomalies in rabbits and no adverse effects on fetuses in rats. Increased incidence of delayed or incomplete ossifications of the metacarpals/metatarsals/phalanges and increased ossification (fused jugal/maxilla bones) were observed in rabbits at 1680 mg/kg. Minor fetal skeletal abnormalities were observed in rats at 3780 mg/kg which was also associated with maternal toxicity including increased incidence of litter loss.
No animal studies have been conducted to evaluate the potential reproductive and embryo-fetal effects of Vancomycin Injection.
8.2 Lactation
Risk Summary
There are insufficient data to inform the levels of vancomycin in human milk. There are no data on the effects of vancomycin on the breastfed infant or milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for vancomycin and any potential adverse effects on the breastfed infant from vancomycin or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Perform a pregnancy test in females of reproductive potential prior to prescribing this formulation of vancomycin [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
8.4 Pediatric Use
Vancomycin Injection is indicated in pediatric patients (1 month and older) [see Indications and Usage (1.1 to 1.5) and Dosage and Administration (2.2)]. In pediatric patients, monitor vancomycin serum concentration and renal function when administering Vancomycin Injection [see Dosage and Administration (2.2, 2.3) and Warnings and Precautions (5.3)]. More severe infusion related reactions related to vancomycin administration may occur in pediatric patients. Concomitant administration of vancomycin and intravenous anesthetic agents has been associated with erythema and histamine-like flushing in all patients including pediatric patients [see Warnings and Precautions (5.2)].
8.5 Geriatric Use
Vancomycin is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection [see Dosage and Administration (2.2)], and it may be useful to monitor renal function [see Warnings and Precautions (5.3)].
-
10 OVERDOSAGE
Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed by dialysis. Hemofiltration and hemoperfusion with polysulfone resin have been reported to result in increased vancomycin clearance.
For current information on the management of overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
-
11 DESCRIPTION
Vancomycin Injection, in single-dose flexible bags contain vancomycin as vancomycin hydrochloride. It is a tricyclic glycopeptide antibacterial drug derived from Amycolatopsis orientalis (formerly Nocardia orientalis). The molecular formula is C66H75Cl2N9O24∙HCl and the molecular weight is 1,485.71. The chemical name is (Sa)-(3S,6R,7R,22R,23S,26S,36R,38aR)-44-{[2-O-(3-amino-2,3,6-trideoxy-3-C-methyl-α-L-lyxo-hexopyranosyl)-β-D-glucopyranosyl]-oxy}-3-(carbamoylmethyl)-10,19-dichloro-2,3,4,5,6,7,23,24,25,26,36,37,38,38a-tetradecahydro-7,22,28,30,32-pentahydroxy-6-[(2R)-4-methyl-2-(methylamino]valeramido]-2,5,24,38,39-pentaoxo-22H-8,11:18,21-dietheno-23,36(iminometha-no)-13,16:31,35-dimetheno-1H,16H-[1,6,9]-oxadiazacyclohexadecino-[4,5-m][10,2,16]-benzoxa-diazacyclotetracosine-26-carboxylic acid, monohydrochloride. Vancomycin hydrochloride has the following structural formula:
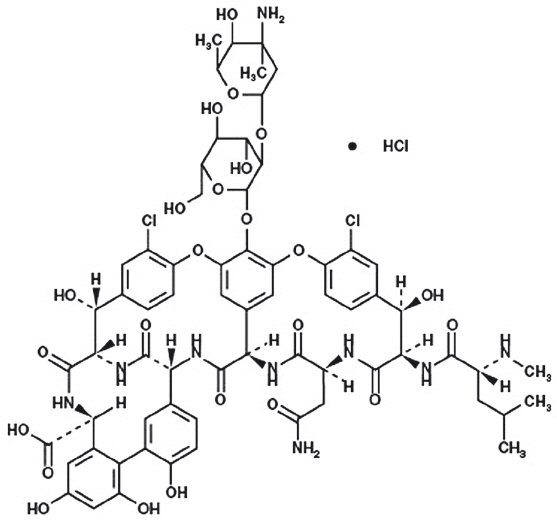
Vancomycin Injection, in single-dose flexible bags are sterile, nonpyrogenic premixed 100 mL, 200 mL, 300 mL or 400 mL solution containing 500 mg, 1 g, 1.5 g or 2 g vancomycin, respectively, as vancomycin hydrochloride. Each 100 mL of solution contains 1.8 mL polyethylene glycol 400, 1.36 g N-acetyl-D-alanine, 1.26 g L-lysine hydrochloride (monochloride) in water for injection. Hydrochloric acid and sodium hydroxide are used for pH adjustment. The pH is 4.5 to 5.5 and the osmolarity is 350 to 475 mOsmol/L.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
In subjects with normal kidney function, multiple intravenous dosing of 1 g of vancomycin (15 mg/kg) infused over 60 minutes produces mean plasma concentrations of approximately 63 mcg/mL immediately after the completion of infusion, mean plasma concentrations of approximately 23 mcg/mL 2 hours after infusion, and mean plasma concentrations of approximately 8 mcg/mL 11 hours after the end of the infusion. Multiple dosing of 500 mg infused over 30 minutes produces mean plasma concentrations of about 49 mcg/mL at the completion of infusion, mean plasma concentrations of about 19 mcg/mL 2 hours after infusion, and mean plasma concentrations of about 10 mcg/mL 6 hours after infusion. The plasma concentrations during multiple dosing are like those after a single dose.
Distribution
The volume of distribution ranges from 0.3 to 0.43 L/kg after intravenous administration.
Vancomycin is approximately 55% serum protein bound as measured by ultrafiltration at vancomycin serum concentrations of 10 to 100 mcg/mL. After intravenous administration of vancomycin, inhibitory concentrations are present in pleural, pericardial, ascitic, and synovial fluids; in urine; in peritoneal dialysis fluid; and in atrial appendage tissue. Vancomycin does not readily diffuse across normal meninges into the spinal fluid; but, when the meninges are inflamed, penetration into the spinal fluid occurs.
Elimination
Mean plasma clearance is about 0.058 L/kg/h, and mean renal clearance is about 0.048 L/kg/h. The mean elimination half-life of vancomycin from plasma is 4 to 6 hours in subjects with normal renal function. In anephric patients, the mean elimination half-life is 7.5 days. Total body and renal clearance of vancomycin may be reduced in the elderly.
Excretion
In the first 24 hours after intravenous administration, about 75% of an administered dose of vancomycin is excreted in urine by glomerular filtration. Renal impairment slows excretion of vancomycin.
About 60% of an intraperitoneal dose of vancomycin administered during peritoneal dialysis is absorbed systemically in 6 hours. Serum concentrations of about 10 mcg/mL are achieved by intraperitoneal injection of 30 mg/kg of vancomycin. However, the safety and efficacy of the intraperitoneal use of vancomycin has not been established in adequate and well-controlled trials [see Warnings and Precautions (5.7)].
12.4 Microbiology
Mechanism of Action
The bactericidal action of vancomycin results primarily from inhibition of cell-wall biosynthesis. In addition, vancomycin alters bacterial-cell-membrane permeability and RNA synthesis.
Resistance
Vancomycin is not active in vitro against gram-negative bacilli, mycobacteria, or fungi. There is no cross-resistance between vancomycin and other antibacterials.
Interaction with Other Antimicrobials
The combination of vancomycin and an aminoglycoside acts synergistically in vitro against many isolates of Staphylococcus aureus, Streptococcus gallolyticus (previously known as Streptococcus bovis), Enterococcus spp, and the viridans group streptococci.
Antimicrobial Activity
Vancomycin has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections [see Indications and Usage (1)].
Aerobic Gram-Positive Bacteria
Corynebacterium spp.
Enterococcus spp. (including Enterococcus faecalis)
Staphylococcus aureus (including methicillin-resistant and methicillin-susceptible isolates)
Coagulase negative staphylococci (including S.epidermidis and methicillin-resistant isolates)
Streptococcus gallolyticus (previously known as Streptococcus bovis)
Viridans group streptococci
The following in vitro data are available, but their clinical significance is unknown.
At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for vancomycin against isolates of similar genus or organism group. However, the efficacy of vancomycin in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
- 13 NONCLINICAL TOXICOLOGY
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Vancomycin Injection is supplied as a ready to use clear, colorless to light brown solution in single-dose flexible bags containing 500 mg, 1 g, 1.5 g, and 2 g vancomycin in 100 mL, 200 mL, 300 mL, and 400 mL of liquid (consists of water and PEG together with the excipients NADA and lysine) [see Description (11)]. The flexible bags are supplied in sealed aluminum overpouches. The bags are supplied in the following packages:
16.2 Storage
NDC number Packaging configuration 70594-041-02 Carton of six 500 mg/100 mL bags 70594-041-03 Carton of twelve 500 mg/100 mL bags 70594-042-02 Carton of six 1 g/200 mL bags 70594-042-03 Carton of twelve 1 g/200 mL bags 70594-043-02 Carton of six 1.5 g/300 mL bags 70594-044-02 Carton of six 2 g/400 mL bags Store below 25°C (77ºF), in original package. Product should be used within 28 days of removal from aluminum overpouch.
-
17 PATIENT COUNSELING INFORMATION
Risk of Embryo-Fetal Toxicity
Advise patients to notify their healthcare provider if they are pregnant prior to treatment with this formulation of vancomycin.
Infusion Reactions During or After Intravenous Use
Advise patients that generalized skin redness, skin rash, itching, flushing, muscle pain, chest pain, shortness of breath, wheezing, or dizziness may occur during Vancomycin Injection infusion. These reactions can be lessened or prevented by infusing the drug over at least 60 minutes.
Acute Kidney Injury
Advise patients that Vancomycin Injection can result in kidney damage and that blood tests are required to monitor vancomycin blood levels and kidney function during therapy.
Hearing Loss or Balance Problems
Advise patients that Vancomycin Injection may result in decreased hearing and to report hearing loss or balance problems to their health care provider.
Antibacterial Resistance
Patients should be counseled that antibacterial drugs including vancomycin, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When vancomycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by vancomycin or other antibacterial drugs in the future.
Diarrhea
Diarrhea is a common problem caused by antibacterial drugs, including vancomycin, which usually ends when the antibacterial drug is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 100 mL Bag Carton
Rx Only
SterileNDC: 70594-041-02
Contains six (6) single-dose
Flexible Bags of NDC: 70594-041-01Vancomycin Injection
500 mg per 100 mL (5 mg/mL)Ready
To UseFor intravenous infusion only. Store below 25°C (77°F), in original package.
Discard unused portion.xellia
PHARMACEUTICALS
-
PRINCIPAL DISPLAY PANEL - 200 mL Bag Carton
Rx Only
SterileNDC: 70594-042-02
Contains six (6) single-dose
Flexible Bags of NDC: 70594-042-01Vancomycin Injection
1 g per 200 mL (5 mg/mL)Ready
To UseFor intravenous infusion only. Store below 25°C (77°F), in original package.
Discard unused portion.xellia
PHARMACEUTICALS
-
PRINCIPAL DISPLAY PANEL - 300 mL Bag Carton
Rx Only
SterileNDC: 70594-043-02
Contains six (6) single-dose
Flexible Bags of NDC: 70594-043-01Vancomycin Injection
1.5 g per 300 mL (5 mg/mL)Ready
To UseFor intravenous infusion only. Store below 25°C (77°F), in original package.
Discard unused portion.xellia
PHARMACEUTICALS
-
PRINCIPAL DISPLAY PANEL - 400 mL Bag Carton
Rx Only
SterileNDC: 70594-044-02
Contains six (6) single-dose
Flexible Bags of NDC: 70594-044-01Vancomycin Injection
2 g per 400 mL (5 mg/mL)Ready
To UseFor intravenous infusion only. Store below 25°C (77°F), in original package.
Discard unused portion.xellia
PHARMACEUTICALS
-
INGREDIENTS AND APPEARANCE
VANCOMYCIN
vancomycin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70594-041 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Vancomycin (UNII: 6Q205EH1VU) (Vancomycin - UNII:6Q205EH1VU) Vancomycin 500 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70594-041-02 6 in 1 CARTON 02/23/2019 1 NDC: 70594-041-01 100 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 70594-041-03 12 in 1 CARTON 02/23/2019 2 NDC: 70594-041-01 100 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211962 02/23/2019 VANCOMYCIN
vancomycin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70594-042 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Vancomycin (UNII: 6Q205EH1VU) (Vancomycin - UNII:6Q205EH1VU) Vancomycin 1 g in 200 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70594-042-02 6 in 1 CARTON 02/23/2019 1 NDC: 70594-042-01 200 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 70594-042-03 12 in 1 CARTON 02/23/2019 2 NDC: 70594-042-01 200 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211962 02/23/2019 VANCOMYCIN
vancomycin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70594-043 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Vancomycin (UNII: 6Q205EH1VU) (Vancomycin - UNII:6Q205EH1VU) Vancomycin 1.5 g in 300 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70594-043-02 6 in 1 CARTON 02/23/2019 1 NDC: 70594-043-01 300 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211962 02/23/2019 VANCOMYCIN
vancomycin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70594-044 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Vancomycin (UNII: 6Q205EH1VU) (Vancomycin - UNII:6Q205EH1VU) Vancomycin 2 g in 400 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70594-044-02 6 in 1 CARTON 02/23/2019 1 NDC: 70594-044-01 400 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211962 02/23/2019 Labeler - Xellia Pharmaceuticals USA LLC (116768762) Registrant - Xellia Pharmaceuticals ApS (305814345)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.