IMLYGIC- talimogene laherparepvec injection, suspension
Imlygic by
Drug Labeling and Warnings
Imlygic by is a Prescription medication manufactured, distributed, or labeled by Amgen Inc, West Pharmaceutical Services, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use IMLYGIC® safely and effectively. See full prescribing information for IMLYGIC.
IMLYGIC (talimogene laherparepvec)
Suspension for intralesional injection
Initial U.S. Approval: 2015
RECENT MAJOR CHANGES
Dosing and Administration, Preparation and Handling (2.2) 10/2019
INDICATIONS AND USAGE
IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery.
Limitations of use: IMLYGIC has not been shown to improve overall survival or have an effect on visceral metastases. (1)
DOSAGE AND ADMINISTRATION
- Administer IMLYGIC by injection into cutaneous, subcutaneous, and/or nodal lesions. (2)
- Recommended starting dose is up to a maximum of 4 mL of IMLYGIC at a concentration of 106 (1 million) plaque-forming units (PFU) per mL. Subsequent doses should be administered up to 4 mL of IMLYGIC at a concentration of 108 (100 million) PFU per mL. (2)
DOSAGE FORMS AND STRENGTHS
Injection: 106 (1 million) PFU per mL, 108 (100 million) PFU per mL in single-use vials (3)
WARNINGS AND PRECAUTIONS
- Accidental Exposure to IMLYGIC: Accidental exposure may lead to transmission of IMLYGIC and herpetic infection. Healthcare providers and close contacts should avoid direct contact with injected lesions, dressings, or body fluids of treated patients. Healthcare providers who are immunocompromised or pregnant should not prepare or administer IMLYGIC. If accidental exposure occurs, exposed individuals should clean the affected area. (5.1)
- Herpetic Infection: Patients who develop herpetic infections should be advised to follow standard hygienic practices to prevent viral transmission. (5.2)
- Injection Site Complications: Consider the risks and benefits before continuing IMLYGIC treatment if persistent infection or delayed healing develops. (5.3)
- Immune-Mediated Events: Consider the risks and benefits of IMLYGIC before initiating treatment in patients who have underlying autoimmune disease or before continuing treatment in patients who develop immune-mediated events. (5.4)
- Plasmacytoma at the Injection Site: Consider the risks and benefits in patients with multiple myeloma or in whom plasmacytoma develops during treatment. (5.5)
- Obstructive Airway Disorder: Use caution when injecting lesions close to major airways. (5.6)
ADVERSE REACTIONS
The most commonly reported adverse drug reactions (≥ 25%) in IMLYGIC-treated patients were fatigue, chills, pyrexia, nausea, influenza-like illness, and injection site pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amgen at 1-855-IMLYGIC (1-855-465-9442) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2019
- Administer IMLYGIC by injection into cutaneous, subcutaneous, and/or nodal lesions. (2)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation and Handling
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Immunocompromised Patients
4.2 Pregnant Patients
5 WARNINGS AND PRECAUTIONS
5.1 Accidental Exposure to IMLYGIC
5.2 Herpetic Infection
5.3 Injection Site Complications
5.4 Immune-Mediated Events
5.5 Plasmacytoma at the Injection Site
5.6 Obstructive Airway Disorder
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1
INDICATIONS AND USAGE
IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery.
Limitations of use: IMLYGIC has not been shown to improve overall survival or have an effect on visceral metastases.
-
2
DOSAGE AND ADMINISTRATION
For intralesional injection only. Do not administer intravenously.
2.1 Dose
Administer IMLYGIC by injection into cutaneous, subcutaneous, and/or nodal lesions that are visible, palpable, or detectable by ultrasound guidance.
IMLYGIC is provided in single-use vials of 1 mL each in two different dose strengths:
- 106 (1 million) plaque-forming units (PFU) per mL (light green cap) – for initial dose only
- 108 (100 million) PFU per mL (royal blue cap) – for all subsequent doses
Recommended Dose and Schedule
The total injection volume for each treatment visit should not exceed 4 mL for all injected lesions combined. It may not be possible to inject all lesions at each treatment visit or over the full course of treatment. Previously injected and/or uninjected lesion(s) may be injected at subsequent treatment visits. The initial recommended dose is up to 4 mL of IMLYGIC at a concentration of 106 (1 million) PFU per mL. The recommended dose for subsequent administrations is up to 4 mL of IMLYGIC at a concentration of 108 (100 million) PFU per mL. The recommended dosing schedule for IMLYGIC is shown in Table 1.
Table 1. Recommended Dose and Schedule for IMLYGIC Treatment Treatment Interval Maximum Injection Volume per Treatment
Visit (all lesions combined)Dose Strength Prioritization of Lesions to be Injected Initial – 4 mL 106 (1 million)
PFU per mL- Inject largest lesion(s) first.
- Prioritize injection of remaining lesion(s) based on lesion size until maximum injection volume is reached or until all injectable lesion(s) have been treated.
Second 3 weeks after initial treatment 4 mL 108 (100 million)
PFU per mL- Inject any new lesion(s) (lesions that have developed since initial treatment) first.
- Prioritize injection of remaining lesion(s) based on lesion size until maximum injection volume is reached or until all injectable lesion(s) have been treated.
All subsequent treatments (including reinitiation) 2 weeks after previous treatment 4 mL 108 (100 million)
PFU per mL- Inject any new lesion(s) (lesions that have developed since previous treatment) first.
- Prioritize injection of remaining lesion(s) based on lesion size until maximum injection volume is reached or until all injectable lesion(s) have been treated.
Dose Volume Determination (per Lesion)
Use Table 2 to determine the volume of IMLYGIC injection for each lesion.
Table 2. Determination of IMLYGIC Injection Volume Based on Lesion Size Lesion Size
(longest dimension)Injection Volume > 5 cm up to 4 mL > 2.5 cm to 5 cm up to 2 mL > 1.5 cm to 2.5 cm up to 1 mL > 0.5 cm to 1.5 cm up to 0.5 mL ≤ 0.5 cm up to 0.1 mL When lesions are clustered together, inject them as a single lesion according to Table 2.
Continue IMLYGIC treatment for at least 6 months unless other treatment is required or until there are no injectable lesions to treat.
Reinitiate IMLYGIC treatment if new unresectable cutaneous, subcutaneous, or nodal lesions appear after a complete response.
2.2 Preparation and Handling
Healthcare providers who are immunocompromised or pregnant should not prepare or administer IMLYGIC and should not come into direct contact with the IMLYGIC injection sites, dressings, or body fluids of treated patients [see Warnings and Precautions (5.1)].
Avoid accidental exposure to IMLYGIC and follow below instructions for preparation, administration, and handling of IMLYGIC:
- Wear personal protective equipment (protective gown or laboratory coat, safety glasses or face shield, and gloves) while preparing or administering IMLYGIC.
- Avoid accidental exposure to IMLYGIC, especially contact with skin, eyes, and mucous membranes.
○ Cover any exposed wounds before handling.
○ In the event of an accidental occupational exposure (e.g., through a splash to the eyes or mucous membranes), flush with clean water for at least 15 minutes.
○ In the event of exposure to broken skin or needle stick, clean the affected area thoroughly with soap and water and/or a disinfectant.
- Clean all surfaces that may have come in contact with IMLYGIC and treat all IMLYGIC spills with a virucidal agent such as 1% sodium hypochlorite or 70% isopropyl alcohol and blot using absorbent materials.
- Dispose of all materials that may have come in contact with IMLYGIC (e.g., vial, syringe, needle, cotton gauze, gloves, masks, or dressings) as biohazardous waste.
- Advise patients to place used dressings and cleaning materials into a sealed plastic bag and dispose in household waste.
Thawing IMLYGIC Vials
1. Determine the total volume required for injection, up to 4 ml [see Dosage and Administration (2.1)].
2. Thaw frozen IMLYGIC vials at room temperature [20º to 25ºC (68º to 77ºF)] until IMLYGIC is liquid (approximately 30 minutes). Do not expose the vial to higher temperatures. Keep the vial in original carton during thawing.
3. Swirl gently. Do NOT shake.
4. After thawing, administer IMLYGIC immediately or store in its original vial and carton, as follows:
Thawed IMLYGIC is stable when stored at temperatures of 2°C (36°F) up to 25°C (77°F) protected from light in its original vial and carton for the storage times specified in Table 3. Do not exceed the storage times specified in Table 3.
IMLYGIC must not be refrozen once it is thawed. Discard any thawed IMLYGIC in the vial stored longer than the specified times in Table 3.
Table 3. Maximum Storage Time for Thawed Imlygic in Vial 106 (1 million) PFU/mL 108 (100 million) PFU/mL 2°C to 8°C (36°F to 46°F) 24 hours 1 week (7 days) up to 25°C (77°F) 12 hours 24 hours 5. Prepare sterile syringes and needles. A detachable 18-26G needle or nondetachable 22-26G staked needle (which minimizes hold up volume) may be used for IMLYGIC withdrawal and a detachable or nondetachable staked needle of 22–26G may be used for injection. Small unit syringes (e.g., 0.5 mL insulin syringes) are recommended for better injection control.
6. Using aseptic technique, remove the vial cap and withdraw the product from the vial into the syringe(s), noting the total volume. Avoid generating aerosols when loading syringes with product and use a biologic safety cabinet if available.
2.3 Administration
Follow the steps below to administer IMLYGIC to patients:
Pre-Injection
1. Clean the lesion and surrounding areas with an alcohol swab and let dry.
2. Treat the injection site with a topical or local anesthetic agent, if necessary. Do not inject anesthetic agent directly into the lesion. Inject anesthetic agent around the periphery of the lesion.
Injection
1. Inject IMLYGIC intralesionally into cutaneous, subcutaneous, and/or nodal lesions that are visible, palpable, or detectable by ultrasound guidance. Using a single insertion point, inject IMLYGIC along multiple tracks as far as the radial reach of the needle allows within the lesion to achieve even and complete dispersion. Multiple insertion points may be used if a lesion is larger than the radial reach of the needle.
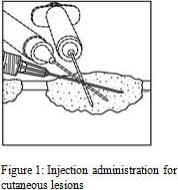
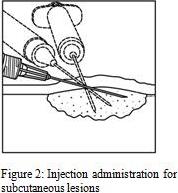
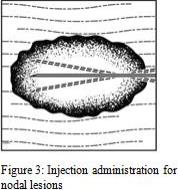
2. Inject IMLYGIC evenly and completely within the lesion by pulling the needle back without exiting the lesion. Redirect the needle as many times as necessary while injecting the remainder of the dose of IMLYGIC. Continue until the full dose is evenly and completely dispersed.
3. When removing the needle, withdraw it from the lesion slowly to avoid leakage of IMLYGIC at the insertion point.
4. Repeat steps 1-2 under pre-injection and steps 1-3 under injection for other lesions to be injected.
5. Use a new needle any time the needle is completely removed from a lesion and each time a different lesion is injected.
Post-Injection
- Apply pressure to the injection site(s) with sterile gauze for at least 30 seconds.
- Swab the injection site(s) and surrounding area with alcohol.
- Change gloves and cover the injected lesion(s) with an absorbent pad and dry occlusive dressing.
- Wipe the exterior of occlusive dressing with alcohol.
- Advise patients to:
- Keep the injection site(s) covered for at least the first week after each treatment visit or longer if the injection site is weeping or oozing.
- Replace the dressing if it falls off.
- 106 (1 million) plaque-forming units (PFU) per mL (light green cap) – for initial dose only
- 3 DOSAGE FORMS AND STRENGTHS
- SPL UNCLASSIFIED SECTION
-
4
CONTRAINDICATIONS
4.1 Immunocompromised Patients
IMLYGIC is a live, attenuated herpes simplex virus and may cause life-threatening disseminated herpetic infection in patients who are immunocompromised. Do not administer IMLYGIC to immunocompromised patients, including those with a history of primary or acquired immunodeficient states, leukemia, lymphoma, AIDS or other clinical manifestations of infection with human immunodeficiency viruses, and those on immunosuppressive therapy [see Nonclinical Toxicology (13.2)].
-
5
WARNINGS AND PRECAUTIONS
5.1 Accidental Exposure to IMLYGIC
Accidental exposure may lead to transmission of IMLYGIC and herpetic infection. Accidental needle stick and splashback to the eyes have been reported in healthcare providers during preparation and administration of IMLYGIC.
Healthcare providers, close contacts (household members, caregivers, sex partners, or persons sharing the same bed), pregnant women, and newborns should avoid direct contact with injected lesions, dressings, or body fluids of treated patients [see Dosage and Administration (2.2)]. Healthcare providers who are immunocompromised or pregnant should not prepare or administer IMLYGIC.
Caregivers should wear protective gloves when assisting patients in applying or changing occlusive dressings and observe safety precautions for disposal of used dressings, gloves, and cleaning materials [see Dosage and Administration (2.2)].
In the event of an accidental exposure to IMLYGIC, exposed individuals should clean the affected area thoroughly with soap and water and/or a disinfectant. If signs or symptoms of herpetic infection develop, the exposed individuals should contact their healthcare provider for appropriate treatment [see Warnings and Precautions (5.2)].
Patients should avoid touching or scratching injection sites or their occlusive dressings, as doing so could lead to inadvertent transfer of IMLYGIC to other areas of the body.
5.2 Herpetic Infection
In clinical studies, herpetic infections (including cold sores and herpetic keratitis) have been reported in patients treated with IMLYGIC. Disseminated herpetic infection may also occur in immunocompromised patients [see Contraindications (4.1)].
Patients who develop suspicious herpes-like lesions should follow standard hygienic practices to prevent viral transmission. Patients or close contacts with suspected herpetic infections should also contact their healthcare provider to evaluate the lesions. Suspected herpetic lesions should be reported to Amgen at 1-855-IMLYGIC (1-855-465-9442); patients or close contacts have the option of follow-up testing for further characterization of the infection.
IMLYGIC is sensitive to acyclovir. Acyclovir or other antiviral agents may interfere with the effectiveness of IMLYGIC. Therefore, consider the risks and benefits of IMLYGIC treatment before administering antiviral agents to manage herpetic infection.
5.3 Injection Site Complications
Necrosis or ulceration of tumor tissue may occur during IMLYGIC treatment. Cellulitis and systemic bacterial infection have been reported in clinical studies. Careful wound care and infection precautions are recommended, particularly if tissue necrosis results in open wounds.
In clinical studies, impaired healing at the injection site has been reported. IMLYGIC may increase the risk of impaired healing in patients with underlying risk factors (e.g., previous radiation at the injection site or lesions in poorly vascularized areas). One patient had an amputation of a lower extremity 6 months after IMLYGIC injection due to an infected non-healing wound. This wound area had been treated with surgery and radiation prior to IMLYGIC treatment and had previous wound complications.
If there is persistent infection or delayed healing of the injection site(s), consider the risks and benefits of IMLYGIC before continuing treatment with IMLYGIC.
5.4 Immune-Mediated Events
IMLYGIC may result in immune-mediated events. In clinical studies, immune-mediated events, including glomerulonephritis, vasculitis, pneumonitis, worsening psoriasis, and vitiligo have been reported in patients treated with IMLYGIC.
Consider the risks and benefits of IMLYGIC before initiating treatment in patients who have underlying autoimmune disease or before continuing treatment in patients who develop immune-mediated events.
5.5 Plasmacytoma at the Injection Site
In a clinical study, a plasmacytoma has been reported in proximity to the injection site after administration of IMLYGIC in a patient with smoldering multiple myeloma.
Consider the risks and benefits of IMLYGIC in patients with multiple myeloma or in whom plasmacytoma develops during treatment.
-
6
ADVERSE REACTIONS
The most commonly reported adverse drug reactions (≥ 25%) in IMLYGIC-treated patients were fatigue, chills, pyrexia, nausea, influenza-like illness, and injection site pain.
The following adverse reactions are discussed in greater detail in another section of the label:
- Herpetic Infection [see Warnings and Precautions (5.2)]
- Injection Site Complications [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of IMLYGIC was evaluated in 419 patients who received at least 1 dose of either IMLYGIC (n = 292) or subcutaneously administered granulocyte-macrophage colony-stimulating factor (GM-CSF) (n = 127) in an open-label, randomized clinical study of patients with stage IIIB, IIIC, and IV melanoma that was not considered to be surgically resectable [see Clinical Studies (14)]. The median duration of exposure to IMLYGIC was 23 weeks (5.3 months). Twenty-six patients were exposed to IMLYGIC for at least 1 year.
Most adverse reactions reported were mild or moderate in severity and generally resolved within 72 hours. The most common grade 3 or higher adverse reaction was cellulitis [see Warnings and Precautions (5.3)].
Pyrexia, chills, and influenza-like illness can occur any time during IMLYGIC treatment but were more frequent during the first 3 months of treatment.
Table 4 below lists adverse reactions with a 5% or greater incidence in the IMLYGIC arm compared to the GM-CSF arm in the clinical study [see Clinical Studies (14)].
Table 4. Adverse Reactions Reported with At Least a 5% Greater Incidence in Patients Treated with IMLYGIC Compared to GM-CSF IMLYGIC
(n = 292)GM-CSF
(n = 127)Adverse Reactions Any Grade
n (%)Grade 3
n (%)Any Grade
n (%)Grade 3
n (%)General disorders and administration site conditions Fatigue 147 (50.3) 6 (2.1) 46 (36.2) 1 (< 1) Chills 142 (48.6) 11 (8.7) Pyrexia 125 (42.8) 11 (8.7) Influenza-like illness 89 (30.5) 2 (< 1) 19 (15.0) Injection site pain 81 (27.7) 2 (< 1) 8 (6.3) Gastrointestinal disorders Nausea 104 (35.6) 1 (< 1) 25 (19.7) Vomiting 62 (21.2) 5 (1.7) 12 (9.5) Diarrhea 55 (18.8) 1 (< 1) 14 (11.0) Constipation 34 (11.6) 8 (6.3) 1 (< 1) Abdominal pain 26 (8.9) 2 (< 1) 3 (2.4) Musculoskeletal and connective tissue disorders Myalgia 51 (17.5) 1 (< 1) 7 (5.5) Arthralgia 50 (17.1) 2 (< 1) 11 (8.7) Pain in extremity 48 (16.4) 4 (1.4) 12 (9.5) 1 (< 1) Nervous system disorders Headache 55 (18.8) 2 (< 1) 12 (9.5) Dizziness 28 (9.6) 4 (3.2) Respiratory, thoracic, and mediastinal disorders Oropharyngeal pain 17 (5.8) 1 (< 1) Investigations Weight decreased 17 (5.8) 1 (< 1) 1 (< 1) Other adverse reactions associated with IMLYGIC in the open-label, randomized study include rash, dermatitis, glomerulonephritis, vitiligo, worsening psoriasis, cellulitis, pneumonitis, vasculitis, herpetic keratitis, obstructive airway disorder, plasmacytoma at the injection site, deep vein thrombosis, and oral herpes.
- Herpetic Infection [see Warnings and Precautions (5.2)]
- 7 DRUG INTERACTIONS
-
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Adequate and well-controlled studies with IMLYGIC have not been conducted in pregnant women. No effects on embryo-fetal development have been observed in a study conducted in pregnant mice. The design of the study limits application of the animal data to humans [see Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
If the patient becomes pregnant while taking IMLYGIC, the patient should be apprised of the potential hazards to the fetus and neonate. Women of childbearing potential should be advised to use an effective method of contraception to prevent pregnancy during treatment with IMLYGIC.
If a pregnant woman has an infection with wild-type Herpes Simplex Virus Type 1 (HSV-1) (primary or reactivation), there is potential for the virus to cross the placental barrier and also a risk of transmission during birth due to viral shedding. Infections with wild-type HSV-1 have been associated with serious adverse effects, including multi-organ failure and death, if a fetus or neonate contracts the wild-type herpes infection. While there are no clinical data to date on IMLYGIC infections in pregnant women, there could be a risk to the fetus or neonate if IMLYGIC were to act in the same manner.
Data
Animal Data
No effects on embryo-fetal development were observed when IMLYGIC was intravenously administered during organogenesis to immunocompetent pregnant mice at doses up to 4 x 108 (400 million) PFU per kg (60-fold higher, on a PFU per kg basis, compared to the maximum clinical dose). Levels of IMLYGIC DNA in pooled fetal blood were at or below the assay detection level. Study design limitations included: 1) administration of IMLYGIC expressing human granulocyte-macrophage colony-stimulating factor (huGM-CSF), which is not biologically active in mice; 2) unknown transplacental kinetics of IMLYGIC following intravenous administration in pregnant mice; and 3) unknown significance of IMLYGIC dose extrapolation from animal to human based on body weight.
8.2 Lactation
Risk Summary
There is no information regarding the presence of IMLYGIC in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for IMLYGIC and any potential adverse effects on the breastfed infant from IMLYGIC or from the underlying maternal condition.
Clinical Considerations
Because medicinal products can be found in human milk, a decision should be made whether to discontinue nursing or to discontinue IMLYGIC while nursing.
8.3 Females and Males of Reproductive Potential
No nonclinical or clinical studies were performed to evaluate the effect of IMLYGIC on fertility.
8.4 Pediatric Use
Safety and effectiveness of IMLYGIC have not been established in pediatric patients.
8.5 Geriatric Use
In clinical studies, no overall differences in safety or efficacy were observed between geriatric patients (≥ 65 years old) and younger patients.
-
10
OVERDOSAGE
There is no clinical experience with an overdose with IMLYGIC. Doses up to 4 mL at dose strength of 108 (100 million) PFU per mL every 2 weeks (maximum cumulative dose of 222.5 x 108 PFU) have been administered in clinical studies, with no evidence of dose-limiting toxicity. The maximum dose of IMLYGIC that can be safely administered has not been determined. In the event of a suspected overdose, the patient should be treated symptomatically and supportive measures instituted as required [see Warnings and Precautions (5)].
-
11
DESCRIPTION
IMLYGIC (talimogene laherparepvec) is a sterile suspension for intralesional injection. IMLYGIC is a live, attenuated HSV-1 that has been genetically modified to express huGM-CSF. The parental virus for IMLYGIC was a primary isolate, which was subsequently altered using recombinant methods to result in gene deletions and insertions.
Each vial contains 1 mL deliverable volume of IMLYGIC at either 1 x 106 (1 million) PFU per mL or 1 x 108 (100 million) PFU per mL concentrations and the following excipients: di-sodium hydrogen phosphate dihydrate (15.4 mg), sodium dihydrogen phosphate dihydrate (2.44 mg), sodium chloride (8.5 mg), myo-inositol (40 mg), sorbitol (20 mg), and water for injection.
The 106 (1 million) PFU per mL vial of IMLYGIC contains a clear to semi-translucent liquid following thaw from its frozen state. The 108 (100 million) PFU per mL vial of IMLYGIC contains a semi-translucent to opaque liquid following thaw from its frozen state. The liquid in each vial may contain white, visible, variously shaped, virus-containing particles.
Each vial of IMLYGIC may also contain residual components of VERO cells including DNA and protein and trace quantities of fetal bovine serum.
The product contains no preservative.
-
12
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
IMLYGIC has been genetically modified to replicate within tumors and to produce the immune stimulatory protein GM-CSF. IMLYGIC causes lysis of tumors, followed by release of tumor-derived antigens, which together with virally derived GM-CSF may promote an antitumor immune response. However, the exact mechanism of action is unknown.
12.3 Pharmacokinetics
Biodistribution (within the body) and Viral Shedding (excretion/secretion)
IMLYGIC viral DNA levels in various tissues and secretions were determined using a quantitative polymerase chain reaction (qPCR) assay. Infectious IMLYGIC at the injection sites and at some potential herpetic lesions was also quantified using viral infectivity assays.
Nonclinical data
Following repeat intratumoral administration in mice, IMLYGIC DNA was primarily detected in the tumor, blood, spleen, lymph node, liver, heart, and kidney. IMLYGIC DNA was not detected in bone marrow, eyes, lachrymal glands, nasal mucosa, or feces. The highest level of IMLYGIC DNA was found in the injected tumor. IMLYGIC DNA was found in the injected tumor through 84 days and in blood samples through 14 days after the last administration of IMLYGIC.
Clinical data
The biodistribution and shedding of intralesionally administered IMLYGIC were investigated in a clinical study that measured IMLYGIC DNA in blood, urine, injection site, occlusive dressings, oral mucosa, anogenital area, and suspected herpetic lesions. Occlusive dressings samples were collected during treatment. Blood and urine samples were collected during treatment and for up to 30 days after the end of treatment. Injection site, oral mucosa, and anogenital area samples were collected during treatment and for up to 60 days after the end of treatment. Suspected herpetic lesion samples were collected any time a patient experienced lesions of suspected herpetic origin. If the qPCR testing for IMLYGIC DNA was positive, then a TCID50 assay was performed to measure viral infectivity. In the 60 patients with melanoma who received IMLYGIC intralesional injection at a dose and schedule same as the clinical study [see Clinical Studies (14)], data indicate that IMLYGIC DNA was present in all sites during the study (see Table 5). .
Table 5. Patients with detectable DNA during treatment Body Fluid/Site Patients with detectable DNA during treatment (n=60) Injection site 60 (100%) Blood 59 (98%) Urine 19 (32%) Injection site 60 (100%) Occlusive dressing 48 (80%) Oral mucosa 8 (13%) Anogenital area 5 (out of 26)a aFor the anogenital area, 26 patients were tested. The proportion of samples and subjects with IMLYGIC DNA was highest during cycle 2 of treatment for the blood, urine, injection site, and occlusive dressings; highest in cycle 1 of treatment for the oral mucosa; and highest in cycles 1 and 2 for the anogenital area. Among patients with detectable IMLYGIC DNA in the blood, urine, oral mucosa and anogenital area, no samples had detectable IMLYGIC DNA 30 days after the end of treatment. For patients with detectable DNA in injected lesions, no samples had detectable IMLYGIC DNA 60 days after the end of treatment.
Overall 3 of 19 patients with lesions of suspected herpetic origin had IMLYGIC DNA present at any time during the study. Of the three subjects who had lesions of suspected herpetic origin that had IMLYGIC DNA detected during the study, none of the suspected lesions were injected with IMLYGIC. None of the subjects with the lesions of the suspected herpetic origin with IMLYGIC DNA had disseminated lesions or received anti-viral therapy and these events were non-serious, lasting from 16 days to 4 months. The data suggest that IMLYGIC may cause herpetic lesions in treated patients.
In addition to the presence of IMLYGIC DNA, viral activity was measured in samples that were positive for IMLYGIC DNA from the injection site, occlusive dressings, oral mucosa, anogenital area, and suspected herpetic lesions. No viral activity was detected in samples of the occlusive dressings, oral mucosa, anogenital area, and suspected herpetic lesions. Infectious IMLYGIC virus was detected at the site of injection in 7 (11%) patients at multiple time points in the study; no samples were positive for viral infectivity after cycle 2 or after the end of treatment.
-
13
NONCLINICAL TOXICOLOGY
13.2 Animal Toxicology and/or Pharmacology
Repeated intratumoral administration at 2 x 108 (200 million) PFU per kg (30-fold maximum proposed clinical dose, extrapolated based on body weight) did not demonstrate any adverse effects in immunocompetent mice. Severe combined immunodeficient (SCID) mice administered repeat intratumoral injections of IMLYGIC at a dose of 30-fold maximum proposed clinical dose developed systemic viral infection (viral inclusion bodies or necrosis in enteric neurons in the gastrointestinal tract, adrenal gland, skin, pancreatic islet cells, eye, pineal gland, and brain).
-
14
CLINICAL STUDIES
The safety and efficacy of intralesional injections of IMLYGIC compared with subcutaneously administered GM-CSF was evaluated in a multicenter, open-label, randomized clinical study in patients with stage IIIB, IIIC, and IV melanoma that was considered to be not surgically resectable. IMLYGIC was injected into cutaneous, subcutaneous, or nodal melanoma lesions and was not injected into visceral lesions. Previous systemic treatment for melanoma was allowed. Patients with active cerebral metastases, bony metastases, extensive visceral disease, primary ocular or mucosal melanoma, evidence of immunosuppression, or receiving treatment with a systemic antiherpetic agent were excluded from the study.
The study included 250 (57%) men and 186 (43%) women. The mean age was 63 (range: 22 to 94) years. Most patients (98%) were white. Seventy percent (70%) of patients had baseline Eastern Cooperative Oncology Group (ECOG) performance status of zero. Seventy percent (70%) of patients had stage IV disease (27% M1a; 21% M1b; and 22% M1c), and 30% had stage III disease. Fifty-three percent (53%) of patients had received prior therapy for melanoma (other than or in addition to surgery, adjuvant therapy, or radiation), and 58% were seropositive for wild-type HSV-1 at baseline.
A total of 436 patients were randomized to receive either IMLYGIC (n = 295) or GM-CSF (n = 141). IMLYGIC was administered by intralesional injection at an initial concentration of 106 (1 million) PFU per mL on Day 1, followed by a concentration of 108 (100 million) PFU per mL on Day 21 and every 2 weeks thereafter, at a dose of up to 4 mL per visit. GM-CSF was administered subcutaneously in 28-day cycles, i.e., 125 µg/m2 daily for 14 days followed by 14 days without GM-CSF administration.
Patients were to be treated for at least 6 months or until there were no injectable lesions. During this period, treatment could continue despite an increase in size in existing lesion(s) and/or development of new lesion(s), unless the patient developed intolerable toxicity or the investigator believed that it was in the best interest of the patient to stop treatment or to be given other therapy for melanoma. After 6 months of treatment, patients were to continue treatment until clinically relevant disease progression (i.e., disease progression associated with a decline in performance status and/or alternative therapy was required in the opinion of the investigator), up to 12 months. Patients experiencing a response at 12 months after the start of treatment could continue treatment for up to an additional 6 months, unless there were no remaining injectable lesions or disease progression. All patients were to be followed for survival status for at least 36 months.
The major efficacy outcome was durable response rate (DRR), defined as the percent of patients with complete response (CR) or partial response (PR) maintained continuously for a minimum of 6 months. Tumor responses were determined according to World Health Organization (WHO) response criteria modified to allow patients who developed new lesions or disease progression of existing lesions to continue the treatment and be evaluated later for tumor response.
The DRR was 16.3% in the IMLYGIC arm and 2.1% in the GM-CSF arm in the overall study population. The unadjusted relative risk was 7.6 (95% CI: 2.4, 24.1), with a p-value < 0.0001. The median time to response was 4.1 (range: 1.2 to 16.7) months in the IMLYGIC arm.
There was no statistically significant difference in overall survival (OS) between the IMLYGIC and the GM-CSF arms. The median OS in the overall study population was 22.9 months in the IMLYGIC arm and 19.0 months in the GM-CSF arm (p = 0.116).
-
16
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
- IMLYGIC is provided as a sterile frozen suspension in a single-use, cyclic olefin polymer (COP) plastic resin vial with a chlorobutyl elastomer stopper, aluminum seal, and polypropylene cap in two different presentations:
- Figure 4: Single-use vial permanently inserted into a clear copolyester plastic sleeve
OR - Figure 5: Single-use vial without a clear plastic sleeve
- Each vial contains a retrievable minimal volume of 1 mL.
- The vial cap is color coded:
- 106 (1 million) PFU per mL is light green (NDC: 55513-078-01).
- 108 (100 million) PFU per mL is royal blue (NDC: 55513-079-01).
- 106 (1 million) PFU per mL is light green (NDC: 55513-078-01).
Storage and Handling
- Store and transport IMLYGIC at −90°C to −70°C (−130°F to −94°F).
- Protect IMLYGIC from light.
- Store IMLYGIC in the carton until use.
- Thaw IMLYGIC immediately prior to administration [see Dosage and Administration (2.2)].
- Do not draw IMLYGIC into a syringe until immediately prior to administration [see Dosage and Administration (2.2)].
-
17
PATIENT COUNSELING INFORMATION
Advise patients and/or close contacts to:
-
Read the FDA-approved patient labeling (Medication Guide).
- Follow instructions below to prevent viral transmission [see Warnings and Precautions (5.1)]:
○ Avoid direct contact with injection sites, dressings, or body fluids of patients.
○ Wear gloves when changing dressing.
○ Avoid touching or scratching injection sites.
○ Keep injection sites covered for at least the first week after each treatment visit or longer if the injection site is weeping or oozing. Replace dressing if it falls off.
○ Dispose of used dressings and cleaning materials in household waste in a sealed plastic bag.
- Female patients of childbearing potential should use an effective method of contraception to prevent pregnancy during treatment with IMLYGIC [see Contraindications (4.2) and Use in Specific Populations (8.1)].
- Close contacts who are pregnant or immunocompromised should not change dressings or clean injection sites [see Warnings and Precautions (5.1)].
- In case of accidental exposure to IMLYGIC, clean the exposed area with soap and water and/or a disinfectant. Patients or close contacts with suspected herpetic infections should contact their healthcare provider to evaluate the lesions. Suspected herpetic lesions should be reported to Amgen at 1-855-IMLYGIC (1-855-465-9442); patients or close contacts have the option of follow-up testing for further characterization of the infection [see Warnings and Precautions (5.1) and (5.2)].
IMLYGIC® (talimogene laherparepvec)
Manufactured by:
BioVex, Inc., a subsidiary of Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799Patent: http://pat.amgen.com/Imlygic/
© 2019 Amgen Inc. All rights reserved.
v5 -
Read the FDA-approved patient labeling (Medication Guide).
-
Medication Guide
IMLYGIC® (imm-LY-jik)
(talimogene laherparepvec)
Read the Medication Guide before you start treatment with IMLYGIC and before each IMLYGIC treatment. There may be new information. This Medication Guide does not tell you everything about IMLYGIC. Talk with your healthcare provider if you have any questions about treatment with IMLYGIC.
What is IMLYGIC?
IMLYGIC is a prescription medicine used to treat a type of cancer called melanoma when it is on your skin or in your lymph glands. IMLYGIC is a weakened form of Herpes Simplex Virus Type 1, which is commonly called the cold sore virus. Your healthcare provider will inject IMLYGIC directly into your tumor(s).
IMLYGIC may not help you live longer and may not shrink cancer in your organs (for example, lung or liver).
Who should not get IMLYGIC?
You should not get IMLYGIC if you are pregnant or have a weakened immune system (for example, an immune deficiency, blood or bone marrow cancer, steroid use, or HIV/AIDS).
What should I tell my healthcare provider before I get IMLYGIC?
Before getting IMLYGIC, tell your healthcare provider if you:
- Are taking steroids or other medicines that suppress your immune system.
- Are taking antiviral medicines to treat or prevent herpes, such as acyclovir.
- Have or ever had medical conditions such as:
○ HIV infection or AIDS.
○ Blood or bone marrow cancer.
○ Autoimmune disease.
○ Other medical conditions that can weaken your immune system.
- Have close contact with someone who has a weakened immune system or is pregnant.
- Are pregnant or plan to become pregnant.
○ IMLYGIC may harm your unborn baby.
○ You should not become pregnant during treatment with IMLYGIC.
○ Talk to your healthcare provider about effective birth control methods.
- Are breastfeeding or plan to breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. IMLYGIC may affect the way other medicines work and other medicines may affect how IMLYGIC works.
How is IMLYGIC given?
Your healthcare provider will inject IMLYGIC directly into your tumor(s) with a needle and syringe. You will get a second treatment 3 weeks after the first treatment. After that, you will get treatments every 2 weeks for as long as you have tumor(s). You can get treated for 6 months or longer.
Your healthcare provider will decide which tumor(s) to inject and may not inject every one.
It is important to care for the treatment sites properly so that IMLYGIC does not spread to other people. Your healthcare provider will show you how to do this.
What should I avoid while getting IMLYGIC?
IMLYGIC virus can spread to other areas of your body or to your close contacts (household members, caregivers, sex partners, or persons sharing the same bed).
Do the following to avoid spreading IMLYGIC to other areas of your body or to your close contacts:
- Avoid direct contact between your treatment sites, dressings, or body fluids and close contacts (for example, use condoms when engaging in sexual activity, avoid kissing close contacts if either has an open mouth sore).
- Wear gloves while putting on or changing your dressings.
- Keep treatment sites covered with airtight and watertight dressings for at least 1 week after each treatment (or longer if the treatment site is weeping or oozing).
- If the dressing comes loose or falls off, replace it right away with a clean dressing.
- Place all used dressings and cleaning materials in a sealed plastic bag and throw them away in the garbage.
- Do not touch or scratch the treatment sites.
What are possible side effects of IMLYGIC?
The most common side effects of IMLYGIC include:
- Tiredness
- Chills
- Fever
- Nausea
- Flu-like symptoms
- Pain at treatment site
Tell your doctor right away if you get any of these signs and symptoms of herpes infection:
- Pain, burning, or tingling in a blister around the mouth or genitals or on the fingers or ears
- Eye pain, light sensitivity, discharge from the eyes, or blurry vision
- Weakness in arms or legs
- Extreme drowsiness (feeling sleepy)
- Mental confusion
If you think you have a herpes infection, inform your healthcare provider. You or your healthcare provider should call Amgen at 1-855-IMLYGIC (1-855-465-9442) for follow-up testing if needed.
These are not all the possible side effects of IMLYGIC. Your healthcare provider can give you more detailed information. Tell your healthcare provider if you have any side effects that bother you or that do not go away. You may report side effects to FDA at 1-800-FDA-1088.
What are the ingredients in IMLYGIC?
Active ingredient: talimogene laherparepvec
Inactive ingredients: di-sodium hydrogen phosphate dihydrate, sodium dihydrogen phosphate dihydrate, sodium chloride, myo-inositol, sorbitol, and water for injection
This Medication Guide summarizes the most important information about IMLYGIC. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider for information about IMLYGIC that was written for healthcare professionals.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
IMLYGIC® (talimogene laherparepvec)
Manufactured by:
BioVex, Inc., a subsidiary of Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799Patent: http://pat.amgen.com/Imlygic/
©2017 Amgen Inc. All rights reserved.
Revised:03/2017
v2 - Are taking steroids or other medicines that suppress your immune system.
-
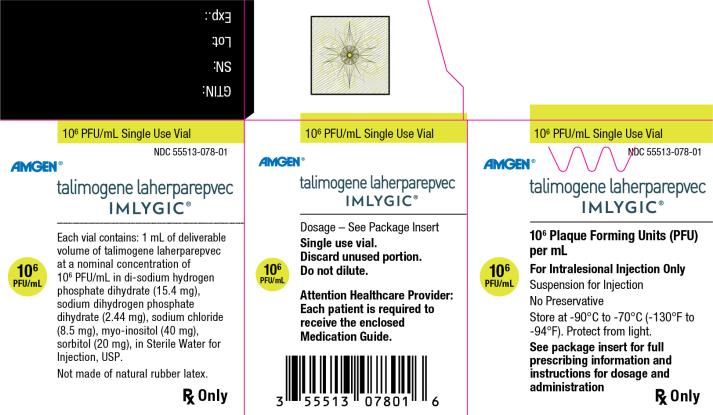
Principal Display Panel
106 PFU/mL Single Use Vial
NDC: 55513-078-01
Amgen®
talimogene laherparepvec
IMLYGIC®
106 Plaque Forming Units (PFU) per mL
106 PFU/mL
For Intralesional Injection Only
Suspension for Injection
No Preservative
Store at -90°C to -70°C (-130°F to -94°F). Protect from light.
See package insert for full prescribing information and instructions for dosage and administration
Rx Only
-
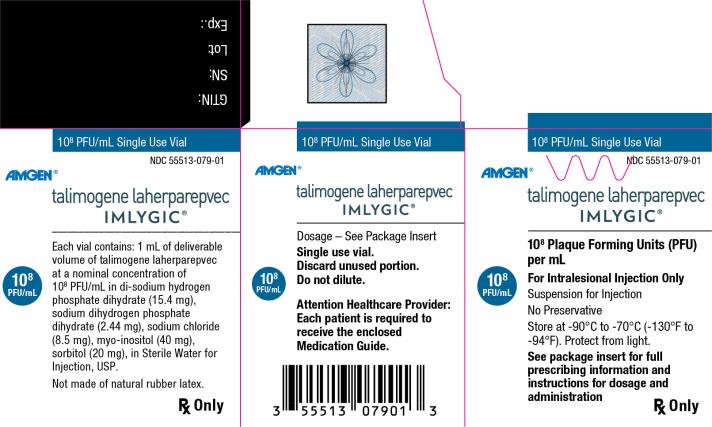
Principal Display Panel
108 PFU/mL Single Use Vial
NDC: 55513-079-01
Amgen®
talimogene laherparepvec
IMLYGIC®
108 Plaque Forming Units (PFU) per mL
108 PFU/mL
For Intralesional Injection Only
Suspension for Injection
No Preservative
Store at -90°C to -70°C (-130°F to -94°F). Protect from light.
See package insert for full prescribing information and instructions for dosage and administration
Rx Only
-
INGREDIENTS AND APPEARANCE
IMLYGIC
talimogene laherparepvec injection, suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55513-078 Route of Administration INTRALESIONAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TALIMOGENE LAHERPAREPVEC (UNII: 07730V90L6) (TALIMOGENE LAHERPAREPVEC - UNII:07730V90L6) TALIMOGENE LAHERPAREPVEC 1000000 [PFU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) 15.4 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) 2.44 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.5 mg in 1 mL INOSITOL (UNII: 4L6452S749) 40.0 mg in 1 mL SORBITOL (UNII: 506T60A25R) 20.0 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55513-078-01 1 in 1 CARTON 11/02/2015 1 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125518 11/02/2015 IMLYGIC
talimogene laherparepvec injection, suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55513-079 Route of Administration INTRALESIONAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TALIMOGENE LAHERPAREPVEC (UNII: 07730V90L6) (TALIMOGENE LAHERPAREPVEC - UNII:07730V90L6) TALIMOGENE LAHERPAREPVEC 100000000 [PFU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) 15.4 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) 2.44 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.5 mg in 1 mL INOSITOL (UNII: 4L6452S749) 40.0 mg in 1 mL SORBITOL (UNII: 506T60A25R) 20.0 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55513-079-01 1 in 1 CARTON 11/02/2015 1 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125518 11/02/2015 Labeler - Amgen Inc (039976196)
Trademark Results [Imlygic]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IMLYGIC 85692936 4889808 Live/Registered |
Amgen Inc. 2012-08-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.