EMEND- aprepitant capsule EMEND- aprepitant kit EMEND- aprepitant powder, for suspension
EMEND by
Drug Labeling and Warnings
EMEND by is a Prescription medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EMEND CAPSULES and EMEND FOR ORAL SUSPENSION safely and effectively. See full prescribing information for EMEND CAPSULES and EMEND FOR ORAL SUSPENSION.
EMEND (aprepitant) capsules, for oral use
EMEND (aprepitant) for oral suspension
Initial U.S. Approval: 2003RECENT MAJOR CHANGES
Indications and Usage (1.2) removal 09/2019 Dosage and Administration (2.2) removal 09/2019 INDICATIONS AND USAGE
EMEND® is a substance P/neurokinin 1 (NK1) receptor antagonist.
EMEND for oral suspension is indicated
- in combination with other antiemetic agents, in patients 6 months of age and older for prevention of:
EMEND capsules is indicated
- in combination with other antiemetic agents, in patients 12 years of age and older for prevention of:
Limitations of Use (1)
- EMEND has not been studied for treatment of established nausea and vomiting.
- Chronic continuous administration of EMEND is not recommended.
DOSAGE AND ADMINISTRATION
Recommended Dosage (2.1)
- EMEND capsules in adults and pediatric patients 12 years of age and older: is 125 mg on Day 1 and 80 mg on Days 2 and 3.
- EMEND for oral suspension in pediatric patients 6 months to less than 12 years of age or pediatric and adult patients unable to swallow capsules: see dosing recommendations in Table 3 in the Full Prescribing Information.
- Administer EMEND 1 hour prior to chemotherapy on Days 1, 2, and 3. If no chemotherapy is given on Days 2 and 3, administer EMEND in morning.
- See Full Prescribing Information for recommended dosages of concomitant dexamethasone and 5-HT3 antagonist for HEC and MEC.
Preparation and Administration (2.2, 2.3)
- EMEND capsules and EMEND for oral suspension can be administered with or without food.
- Swallow EMEND capsules whole.
- EMEND for oral suspension should be prepared by healthcare provider. Once prepared, it may be administered either by a healthcare provider, patient, or caregiver.
- For details on preparation see Full Prescribing Information.
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- CYP3A4 Interactions: Aprepitant is a substrate, weak-to-moderate inhibitor and inducer of CYP3A4; See Full Prescribing Information for recommendations regarding contraindications, risk of adverse reactions, and dosage adjustments of EMEND and concomitant drugs. (4, 5.1, 7.1, 7.2)
- Warfarin (a CYP2C9 substrate): Risk of decreased INR of prothrombin time; monitor INR in 2-week period, particularly at 7 to 10 days, following initiation of EMEND. (5.2, 7.1)
- Hormonal Contraceptives: Efficacy of contraceptives may be reduced during administration of and for 28 days following the last dose of EMEND. Use effective alternative or back-up methods of contraception. (5.3, 7.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (≥3%) are (6.1):
- Adults: fatigue, diarrhea, asthenia, dyspepsia, abdominal pain, hiccups, white blood cell count decreased, dehydration, and alanine aminotransferase increased.
- Pediatrics: neutropenia, headache, diarrhea, decreased appetite, cough, fatigue, hemoglobin decreased, dizziness, and hiccups.
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation Instructions for EMEND for Oral Suspension -- for Healthcare Providers
2.3 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Clinically Significant CYP3A4 Drug Interactions
5.2 Decrease in INR with Concomitant Warfarin
5.3 Risk of Reduced Efficacy of Hormonal Contraceptives
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Aprepitant on the Pharmacokinetics of Other Drugs
7.2 Effect of Other Drugs on the Pharmacokinetics of Aprepitant
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Renal Impairment
8.7 Patients with Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Prevention of Nausea and Vomiting Associated with HEC in Adults
14.2 Prevention of Nausea and Vomiting Associated with MEC in Adults
14.3 Prevention of Nausea and Vomiting Associated with HEC or MEC in Pediatric Patients
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
EMEND® for oral suspension, in combination with other antiemetic agents, is indicated in patients 6 months of age and older for the prevention of:
- acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin.
- nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC).
EMEND® capsules, in combination with other antiemetic agents, is indicated in patients 12 years of age and older for the prevention of:
- acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin.
- nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC).
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Adults and Pediatric Patients 12 Years of Age and Older
The recommended oral dosage of EMEND capsules, dexamethasone, and a 5-HT3 antagonist in adults and pediatric patients 12 years of age and older who can swallow oral capsules, for the prevention of nausea and vomiting associated with administration of HEC or MEC is shown in Table 1 or Table 2, respectively. For patients who cannot swallow oral capsules, EMEND for oral suspension can be used instead of EMEND capsules as shown in Table 3.
Table 1: Recommended Dosing for the Prevention of Nausea and Vomiting Associated with HEC Population Day 1 Day 2 Day 3 Day 4 - * Administer EMEND capsules 1 hour prior to chemotherapy treatment on Days 1, 2, and 3. If no chemotherapy is given on Days 2 and 3, administer EMEND capsules in the morning.
- † Administer dexamethasone 30 minutes prior to chemotherapy treatment on Day 1 and in the morning on Days 2 through 4. A 50% dosage reduction of dexamethasone is recommended to account for a drug interaction with EMEND [see Clinical Pharmacology (12.3)].
EMEND capsules* Adults and Pediatric Patients 12 Years and Older 125 mg orally 80 mg orally 80 mg orally none Dexamethasone Adults 12 mg orally 8 mg orally 8 mg orally 8 mg orally Pediatric Patients 12 Years and Older If a corticosteroid, such as dexamethasone, is co-administered, administer 50% of the recommended corticosteroid dose on Days 1 through 4 [see Clinical Studies (14.3)].† 5-HT3 antagonist Adults and Pediatric Patients 12 Years and Older See selected 5-HT3 antagonist prescribing information for the recommended dosage none none none Table 2: Recommended Dosing for the Prevention of Nausea and Vomiting Associated with MEC Population Day 1 Day 2 Day 3 - * Administer EMEND capsules 1 hour prior to chemotherapy treatment on Days 1, 2, and 3. If no chemotherapy is given on Days 2 and 3, administer EMEND capsules in the morning.
- † Administer dexamethasone 30 minutes prior to chemotherapy treatment on Day 1. A 50% dosage reduction of dexamethasone is recommended to account for a drug interaction with EMEND [see Clinical Pharmacology (12.3)].
EMEND capsules* Adults and Pediatric Patients 12 Years and Older 125 mg orally 80 mg orally 80 mg orally Dexamethasone Adults 12 mg orally none none Pediatric Patients 12 Years and Older If a corticosteroid, such as dexamethasone, is co-administered, administer 50% of the recommended corticosteroid dose on Days 1 through 4 [see Clinical Studies (14.3)].† 5-HT3 antagonist Adults and Pediatric Patients 12 Years and Older See the selected 5-HT3 antagonist prescribing information for recommended dosage none none Pediatric Patients 6 Months to less than 12 Years of Age or Pediatric and Adult Patients Unable to Swallow Capsules
The recommended dose of EMEND for oral suspension to be administered with a 5-HT3 antagonist, with or without a corticosteroid, for the prevention of nausea and vomiting associated with administration of HEC or MEC is specified in Table 3. Dosing of EMEND for oral suspension is based on weight, to a maximum of 125 mg on Day 1 and 80 mg on Days 2 and 3. Dosing in pediatric patients less than 6 kg is not recommended.
Table 3: Recommended Dosing in Pediatric Patients 6 Months to Less than 12 Years of Age or Pediatric and Adult Patients Unable to Swallow Capsules Population Day 1 Day 2 Day 3 Day 4 - * After preparation, the final concentration of EMEND for oral suspension is 25 mg/mL [see Dosage and Administration (2.3)]. Administer EMEND for oral suspension 1 hour prior to chemotherapy treatment on Days 1, 2, and 3. If no chemotherapy is given on Days 2 and 3, administer EMEND for oral suspension in the morning.
- † Administer dexamethasone 30 minutes prior to chemotherapy treatment on Day 1. A 50% dosage reduction of dexamethasone is recommended to account for a drug interaction with EMEND [see Clinical Pharmacology (12.3)].
EMEND for oral suspension* Pediatric Patients 6 Months to Less than12 Years or Pediatric and Adult Patients Unable to Swallow Capsules 3 mg/kg orally Maximum dose 125 mg 2 mg/kg orally Maximum dose 80 mg 2 mg/kg orally Maximum dose 80 mg none Dexamethasone Adults Unable to Swallow Capsules See Table 1 or 2 See Table 1 or 2 See Table 1 or 2 See Table 1 or 2 Pediatric Patients 6 Months to Less than12 Years or Pediatric Patients Unable to Swallow Capsules If a corticosteroid, such as dexamethasone, is co-administered, administer 50% of the recommended corticosteroid dose on Days 1 through 4 [see Clinical Studies (14.3)].† 5-HT3 antagonist Pediatric Patients 6 Months to Less than12 Years or Pediatric and Adult Patients Unable to Swallow Capsules See selected 5-HT3 antagonist prescribing information for the recommended dosage none none none 2.2 Preparation Instructions for EMEND for Oral Suspension -- for Healthcare Providers
EMEND for oral suspension should be prepared by a healthcare provider. Once prepared, it may be administered either by a healthcare provider, patient, or caregiver.
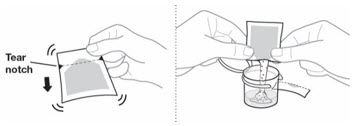
Before preparing EMEND:
- Do not open the pouch of EMEND until ready to prepare the medicine.
- Store the pouch at room temperature [between 68°F-77°F (20°C-25°C)].
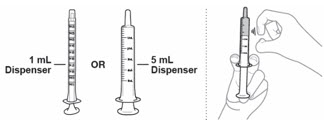
Table 4: Instructions for Healthcare Providers on How to Prepare EMEND for Oral Suspension EMEND for oral suspension is packaged as a kit with one 1 mL oral dosing dispenser, one 5 mL oral dosing dispenser, one cap and one mixing cup. 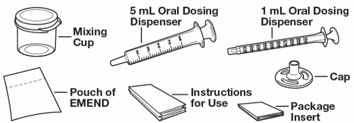
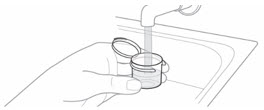
- 1. Fill the mixing cup with room temperature drinking water.
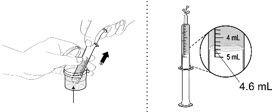
- 2.
Fill the 5 mL oral dosing dispenser with 4.6 mL of water from the mixing cup.
Make sure no air is in the dispenser - if air is present, remove.
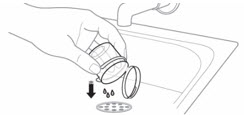
- 3. Discard all the unused water remaining in the mixing cup.
- 4. Add the 4.6 mL of water from the dispenser back into the mixing cup.
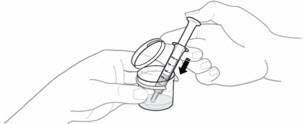
- 5.
Each pouch of EMEND for oral suspension contains 125 mg of aprepitant which is to be suspended in 4.6 mL of water giving a final concentration of 25 mg/mL.
Hold the EMEND for oral suspension pouch upright and shake the contents to the bottom before opening the pouch. - 6. Pour the entire contents of the pouch into the 4.6 mL of water in the mixing cup and snap the lid shut.
- 7.
Mix the EMEND suspension gently by swirling 20 times; then gently invert the mixing cup 5 times.
To prevent foaming, do not shake the mixing cup. The mixture will be cloudy pink to light pink.
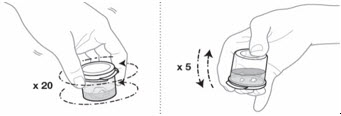
- 8.
Check the EMEND mixture for any clumps or foaming:
- If any clumps are present, repeat Step 7 until there are no clumps.
- If there is any foam, wait for the foam to disappear before going on to Step 9.
- 9.
Fill the dispenser with the prescribed dose shown above in Table 3.
- Choose the dispenser based on dose:
- Use 1 mL dispenser if dose is 1 mL or less.
- Use 5 mL dispenser if dose is more than 1 mL.
- Fill the dispenser with the prescribed dose from the cup.
- If the dose is less than 1 mL round to the nearest 0.1 mL.
- If the dose is more than 1 mL round to the nearest 0.2 mL.
- It is common to have medicine leftover in the cup.
- Choose the dispenser based on dose:
Make sure no air is in the dispenser - if air is present, remove.
Make sure the dispenser contains the prescribed dose.- 10. Place the cap on the dispenser until it clicks.
- 11. If the dose is not administered immediately after measuring, store filled oral dosing dispenser(s) in the refrigerator [between 36°F-46°F (2°C-8°C)] for up to 72 hours prior to use. When dispensing dose(s) to the patient or caregiver, instruct them to refrigerate the oral dosing dispenser(s) until they are ready to administer the dose.
- 12. When ready to use, the mixture can be kept at room temperature [between 68°F-77°F (20°C-25°C)] for up to 3 hours.
- 13. Discard the mixing cup along with any remaining suspension.
2.3 Administration Instructions
EMEND capsules and EMEND for oral suspension can be administered with or without food.
EMEND for oral suspension
- The dose will be prepared by the healthcare provider and dispensed to the patient or caregiver in an oral dispenser.
- Keep the dispenser in the refrigerator until administered to the patient. The dose can be stored at room temperature for up to 3 hours before use.
- When ready to use, take the cap off the dispenser, place the dispenser in the patient's mouth along the inner cheek on either the right or left side. Slowly dispense the medicine.
- The dose must be used within 72 hours of preparation.
- Discard any doses remaining after 72 hours.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
EMEND is contraindicated in patients:
- who are hypersensitive to any component of the product. Hypersensitivity reactions including anaphylactic reactions have been reported [see Adverse Reactions (6.2)].
- taking pimozide. Inhibition of CYP3A4 by aprepitant could result in elevated plasma concentrations of this drug which is a CYP3A4 substrate, potentially causing serious or life-threatening reactions, such as QT prolongation, a known adverse reaction of pimozide [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Clinically Significant CYP3A4 Drug Interactions
Aprepitant is a substrate, a weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4.
- Use of EMEND with other drugs that are CYP3A4 substrates, may result in increased plasma concentration of the concomitant drug.
- Use of pimozide with EMEND is contraindicated due to the risk of significantly increased plasma concentrations of pimozide, potentially resulting in prolongation of the QT interval, a known adverse reaction of pimozide [see Contraindications (4)].
- Use of EMEND with strong or moderate CYP3A4 inhibitors (e.g., ketoconazole, diltiazem) may increase plasma concentrations of aprepitant and result in an increased risk of adverse reactions related to EMEND.
- Use of EMEND with strong CYP3A4 inducers (e.g., rifampin) may result in a reduction in aprepitant plasma concentrations and decreased efficacy of EMEND.
See Table 8 and Table 9 for a listing of potentially significant drug interactions [see Drug Interactions (7.1, 7.2)].
5.2 Decrease in INR with Concomitant Warfarin
Coadministration of EMEND with warfarin, a CYP2C9 substrate, may result in a clinically significant decrease in International Normalized Ratio (INR) of prothrombin time [see Clinical Pharmacology (12.3)]. Monitor the INR in patients on chronic warfarin therapy in the 2-week period, particularly at 7 to 10 days, following initiation of the 3-day regimen of EMEND with each chemotherapy cycle [see Drug Interactions (7.1)].
5.3 Risk of Reduced Efficacy of Hormonal Contraceptives
Upon coadministration with EMEND, the efficacy of hormonal contraceptives may be reduced during administration of and for 28 days following the last dose of EMEND [see Clinical Pharmacology (12.3)]. Advise patients to use effective alternative or back-up methods of contraception during treatment with EMEND and for 1 month following the last dose of EMEND [see Drug Interactions (7.1), Use in Specific Populations (8.3)].
- Use of EMEND with other drugs that are CYP3A4 substrates, may result in increased plasma concentration of the concomitant drug.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The overall safety of EMEND was evaluated in approximately 6800 individuals.
Adults
In 2 active-controlled, double-blind clinical trials in patients receiving highly emetogenic chemotherapy (HEC) (Studies 1 and 2), EMEND in combination with ondansetron and dexamethasone (EMEND regimen) was compared to ondansetron and dexamethasone alone (standard therapy) [see Clinical Studies (14.1)].
In 2 active-controlled clinical trials in patients receiving moderately emetogenic chemotherapy (MEC) (Studies 3 and 4), EMEND in combination with ondansetron and dexamethasone (EMEND regimen) was compared to ondansetron and dexamethasone alone (standard therapy) [see Clinical Studies (14.2)]. The most common adverse reaction reported in patients who received MEC in pooled Studies 3 and 4 was dyspepsia (6% versus 4%).
Across these 4 studies there were 1412 patients treated with the EMEND regimen during Cycle 1 of chemotherapy and 1099 of these patients continued into the Multiple-Cycle extension for up to 6 cycles of chemotherapy. The most common adverse reactions reported in patients who received HEC and MEC in pooled Studies 1, 2, 3 and 4 are listed in Table 5.
Table 5: Most Common Adverse Reactions in Patients Receiving HEC and MEC from a Pooled Analysis of HEC and MEC Studies* EMEND, ondansetron, and dexamethasone†
(N=1412)Ondansetron and dexamethasone‡
(N=1396)- * Reported in ≥ 3% of patients treated with the EMEND regimen and at a greater incidence than standard therapy.
- † EMEND regimen
- ‡ Standard therapy
fatigue 13% 12% diarrhea 9% 8% asthenia 7% 6% dyspepsia 7% 5% abdominal pain 6% 5% hiccups 5% 3% white blood cell count decreased 4% 3% dehydration 3% 2% alanine aminotransferase increased 3% 2% In a pooled analysis of the HEC and MEC studies, less common adverse reactions reported in patients treated with the EMEND regimen are listed in Table 6.
Table 6: Less Common Adverse Reactions in EMEND-Treated Patients from a Pooled Analysis of HEC and MEC Studies* - * Reported in > 0.5% of patients treated with the EMEND regimen, at a greater incidence than standard therapy and not previously described in Table 5.
Infection and Infestations oral candidiasis, pharyngitis Blood and the Lymphatic System Disorders anemia, febrile neutropenia, neutropenia, thrombocytopenia Metabolism and Nutrition Disorders decreased appetite, hypokalemia Psychiatric Disorders anxiety Nervous System Disorders dizziness, dysgeusia, peripheral neuropathy Cardiac Disorders palpitations Vascular Disorders flushing, hot flush Respiratory, Thoracic and Mediastinal Disorders cough, dyspnea, oropharyngeal pain Gastrointestinal Disorders dry mouth, eructation, flatulence, gastritis, gastroesophageal reflux disease, nausea, vomiting Skin and Subcutaneous Tissue Disorders alopecia, hyperhidrosis, rash Musculoskeletal and Connective Tissue Disorders musculoskeletal pain General Disorders and Administration Site Condition edema peripheral, malaise Investigations aspartate aminotransferase increased, blood alkaline phosphatase increased, blood sodium decreased, blood urea increased, proteinuria, weight decreased In an additional active-controlled clinical study in 1169 patients receiving EMEND and HEC, the adverse reactions were generally similar to that seen in the other HEC studies with EMEND.
In another CINV study, Stevens-Johnson syndrome was reported as a serious adverse reaction in a patient receiving the EMEND regimen with cancer chemotherapy.
Adverse reactions in the Multiple-Cycle extensions of HEC and MEC studies for up to 6 cycles of chemotherapy were generally similar to that observed in Cycle 1.
Pediatric Patients 6 Months to 17 Years of Age
In a pooled analysis of 2 active-controlled clinical trials in pediatric patients aged 6 months to 17 years who received highly or moderately emetogenic cancer chemotherapy (Study 5 and a safety study, Study 6), EMEND in combination with ondansetron with or without dexamethasone (EMEND regimen) was compared to ondansetron with or without dexamethasone (control regimen).
There were 184 patients treated with the EMEND regimen during Cycle 1 and 215 patients received open-label EMEND for up to 9 additional cycles of chemotherapy.
In Cycle 1, the most common adverse reactions reported in pediatric patients treated with the EMEND regimen in pooled Studies 5 and 6 are listed in Table 7.
Table 7: Most Common Adverse Reactions in EMEND-Treated Pediatric Patients in HEC and MEC Pooled Studies 5 and 6* EMEND and ondansetron†
(N=184)Ondansetron‡
(N=168)- * Reported in ≥3% of patients treated with the EMEND regimen and at a greater incidence than control regimen.
- † EMEND regimen
- ‡ Control regimen
neutropenia 13% 11% headache 9% 5% diarrhea 6% 5% decreased appetite 5% 4% cough 5% 3% fatigue 5% 2% hemoglobin decreased 5% 4% dizziness 5% 1% hiccups 4% 1% Forty-nine patients were treated with ifosfamide chemotherapy in each arm. Two of the patients treated with ifosfamide in the aprepitant arm developed behavioral changes (agitation = 1; abnormal behavior = 1), whereas no patient treated with ifosfamide in the control arm developed behavioral changes. Aprepitant has the potential for increasing ifosfamide-mediated neurotoxicity through induction of CYP3A4 [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of EMEND. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: pruritus, rash, urticaria, Stevens-Johnson syndrome/toxic epidermal necrolysis.
Immune system disorders: hypersensitivity reactions including anaphylactic reactions [see Contraindications (4)].
Nervous system disorders: ifosfamide-induced neurotoxicity reported after EMEND and ifosfamide coadministration.
-
7 DRUG INTERACTIONS
7.1 Effect of Aprepitant on the Pharmacokinetics of Other Drugs
Aprepitant is a substrate, a weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Aprepitant is also an inducer of CYP2C9 [see Clinical Pharmacology (12.3)].
Aprepitant acts as a moderate inhibitor of CYP3A4 when administered as a 3-day regimen (125-mg/80-mg/80-mg) and can increase plasma concentrations of concomitant drugs that are substrates for CYP3A4. Some substrates of CYP3A4 are contraindicated with EMEND [see Contraindications (4)]. Dosage adjustment of some CYP3A4 and CYP2C9 substrates may be warranted, as shown in Table 8.
Table 8: Effects of Aprepitant on the Pharmacokinetics of Other Drugs CYP3A4 Substrates Pimozide Clinical Impact Increased pimozide exposure. Intervention EMEND is contraindicated [see Contraindications (4)]. Benzodiazepines Clinical Impact Increased exposure to midazolam or other benzodiazepines metabolized via CYP3A4 (alprazolam, triazolam) may increase the risk of adverse reactions [see Clinical Pharmacology (12.3)]. Intervention - Monitor for benzodiazepine-related adverse reactions.
- Depending on the clinical situation (e.g., elderly patients) and degree of monitoring available, reduce the dose of intravenous midazolam
Dexamethasone Clinical Impact Increased dexamethasone exposure [see Clinical Pharmacology (12.3)]. Intervention Reduce the dose of oral dexamethasone by approximately 50% [see Dosage and Administration (2.1)]. Methylprednisolone Clinical Impact Increased methylprednisolone exposure [see Clinical Pharmacology (12.3)]. Intervention - Reduce the dose of intravenous methylprednisolone by approximately 25%
- Reduce the dose of oral methylprednisolone by approximately 50%
Chemotherapeutic agents that are metabolized by CYP3A4 Clinical Impact Increased exposure of the chemotherapeutic agent may increase the risk of adverse reactions [see Clinical Pharmacology (12.3)]. Intervention Vinblastine, vincristine, or ifosfamide or other chemotherapeutic agents - Monitor for chemotherapeutic-related adverse reactions.
- No dosage adjustment needed.
Hormonal Contraceptives Clinical Impact Decreased hormonal exposure during administration of and for 28 days after administration of the last dose of EMEND [see Warnings and Precautions (5.3), Use in Specific Populations (8.3), Clinical Pharmacology (12.3)]. Intervention Effective alternative or back-up methods of contraception (such as condoms and spermicides) should be used during treatment with EMEND and for 1 month following the last dose of EMEND. Examples birth control pills, skin patches, implants, and certain IUDs CYP2C9 Substrates Warfarin Clinical Impact Decreased warfarin exposure and decreased prothrombin time (INR) [see Warnings and Precautions (5.2), Clinical Pharmacology (12.3)]. Intervention In patients on chronic warfarin therapy, monitor the prothrombin time (INR) in the 2-week period, particularly at 7 to 10 days, following initiation of the 3-day EMEND regimen with each chemotherapy cycle. Other 5-HT3 Antagonists Clinical Impact No change in the exposure of the 5-HT3 antagonist [see Clinical Pharmacology (12.3)]. Intervention No dosage adjustment needed Examples ondansetron, granisetron, dolasetron 7.2 Effect of Other Drugs on the Pharmacokinetics of Aprepitant
Aprepitant is a CYP3A4 substrate [see Clinical Pharmacology (12.3)]. Co-administration of EMEND with drugs that are inhibitors or inducers of CYP3A4 may result in increased or decreased plasma concentrations of aprepitant, respectively, as shown in Table 9.
Table 9: Effects of Other Drugs on Pharmacokinetics of Aprepitant Moderate to Strong CYP3A4 Inhibitors Clinical Impact Significantly increased exposure of aprepitant may increase the risk of adverse reactions associated with EMEND [see Adverse Reactions (6.1) and Clinical Pharmacology (12.3)]. Intervention Avoid concomitant use of EMEND. Examples Moderate inhibitor:
diltiazem
Strong inhibitors:
ketoconazole, itraconazole, nefazodone, troleandomycin, clarithromycin, ritonavir, nelfinavirStrong CYP3A4 Inducers Clinical Impact Substantially decreased exposure of aprepitant in patients chronically taking a strong CYP3A4 inducer may decrease the efficacy of EMEND [see Clinical Pharmacology (12.3)]. Intervention Avoid concomitant use of EMEND. Examples rifampin, carbamazepine, phenytoin -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are insufficient data on use of EMEND in pregnant women to inform a drug associated risk. In animal reproduction studies, no adverse developmental effects were observed in rats or rabbits exposed during the period of organogenesis to systemic drug levels (AUC) approximately 1.5 times the adult human exposure at the 125-mg/80-mg/80-mg EMEND regimen [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In embryofetal development studies in rats and rabbits, aprepitant was administered during the period of organogenesis at oral doses up to 1000 mg/kg twice daily in rats and up to the maximum tolerated dose of 25 mg/kg/day in rabbits. No embryofetal lethality or malformations were observed at any dose level in either species. The exposures (AUC) in pregnant rats at 1000 mg/kg twice daily and in pregnant rabbits at 125 mg/kg/day were approximately 1.5 times the adult exposure at the 125-mg/80-mg/80-mg EMEND regimen. Aprepitant crosses the placenta in rats and rabbits.
8.2 Lactation
Risk Summary
Lactation studies have not been conducted to assess the presence of aprepitant in human milk, the effects on the breastfed infant, or the effects on milk production. Aprepitant is present in rat milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EMEND and any potential adverse effects on the breastfed infant from EMEND or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Upon administration of EMEND, the efficacy of hormonal contraceptives may be reduced. Advise females of reproductive potential using hormonal contraceptives to use an effective alternative or back-up non-hormonal contraceptive (such as condoms and spermicides) during treatment with EMEND and for 1 month following the last dose [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
8.4 Pediatric Use
The safety and effectiveness of EMEND for oral suspension have been established in pediatric patients 6 months of age and older and EMEND capsules in pediatric patients 12 years of age and older for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of HEC, including high-dose cisplatin, and MEC. Use of EMEND in these age groups is supported by evidence from 302 pediatric patients in a randomized, double-blind, active comparator controlled clinical study (n = 207 patients aged 6 months to less than 12 years, n = 95 patients aged 12 through 17 years). EMEND was studied in combination with ondansetron with or without dexamethasone (at the discretion of the physician) [see Clinical Studies (14.3)]. Adverse reactions were similar to those reported in adult patients [see Adverse Reactions (6.1)].
The safety and effectiveness of EMEND for the prevention of nausea and vomiting associated with HEC or MEC have not been established in patients less than 6 months.
Juvenile Animal Study
A study was conducted in young rats to evaluate the effects of aprepitant on growth and on neurobehavioral and sexual development. Rats were treated at oral doses up to the maximum feasible dose of 1000 mg/kg twice daily (providing exposure in male rats lower than the exposure at the recommended pediatric human dose and exposure in female rats equivalent to the pediatric human exposure) from the early postnatal period (Postnatal Day 10) through Postnatal Day 58. Slight changes in the onset of sexual maturation were observed in female and male rats; however, there were no effects on mating, fertility, embryonic-fetal survival, or histomorphology of the reproductive organs. There were no effects in neurobehavioral tests of sensory function, motor function, and learning and memory.
8.5 Geriatric Use
Of the 544 adult cancer patients treated with EMEND in CINV clinical studies, 31% were aged 65 and over, while 5% were aged 75 and over. Other reported clinical experience with EMEND has not identified differences in responses between elderly and younger patients. In general, use caution when dosing elderly patients as they have a greater frequency of decreased hepatic, renal or cardiac function and concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
8.6 Patients with Renal Impairment
The pharmacokinetics of aprepitant in patients with severe renal impairment and those with end stage renal disease (ESRD) requiring hemodialysis were similar to those of healthy subjects with normal renal function. No dosage adjustment is necessary for patients with any degree of renal impairment or for patients with ESRD undergoing hemodialysis.
8.7 Patients with Hepatic Impairment
The pharmacokinetics of aprepitant in patients with mild and moderate hepatic impairment were similar to those of healthy subjects with normal hepatic function. No dosage adjustment is necessary for patients with mild to moderate hepatic impairment (Child-Pugh score 5 to 9). There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh score greater than 9). Therefore, additional monitoring for adverse reactions in these patients may be warranted when EMEND is administered [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
No specific information is available on the treatment of overdosage.
Drowsiness and headache were reported in one patient who ingested 1440 mg of EMEND (approximately 11 times the maximum recommended single dose).
In the event of overdose, EMEND should be discontinued and general supportive treatment and monitoring should be provided. Because of the antiemetic activity of EMEND, drug-induced emesis may not be effective in cases of EMEND overdosage.
Aprepitant is not removed by hemodialysis.
-
11 DESCRIPTION
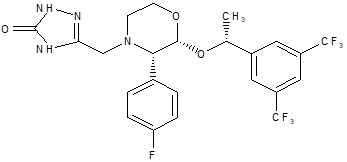
EMEND capsules contain the active ingredient, aprepitant. Aprepitant is a substance P/neurokinin 1 (NK1) receptor antagonist, an antiemetic agent, chemically described as 5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one.
Its empirical formula is C23H21F7N4O3, and its structural formula is:
Aprepitant is a white to off-white crystalline solid, with a molecular weight of 534.43. It is practically insoluble in water. Aprepitant is sparingly soluble in ethanol and isopropyl acetate and slightly soluble in acetonitrile.
Each capsule of EMEND for oral administration contains either 80 mg or 125 mg of aprepitant and the following inactive ingredients: sucrose, microcrystalline cellulose, hydroxypropyl cellulose and sodium lauryl sulfate. The capsule shell excipients are gelatin, titanium dioxide, and may contain sodium lauryl sulfate and silicon dioxide. The 125-mg capsule also contains red ferric oxide and yellow ferric oxide.
Each pouch of EMEND for oral suspension 125 mg contains 125 mg of aprepitant and the following inactive ingredients: sucrose, lactose, hydroxypropyl cellulose, sodium lauryl sulfate, red iron oxide, and sodium stearyl fumarate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Aprepitant is a selective high-affinity antagonist of human substance P/neurokinin 1 (NK1) receptors. Aprepitant has little or no affinity for serotonin (5-HT3), dopamine, and corticosteroid receptors, the targets of existing therapies for chemotherapy-induced nausea and vomiting (CINV).
Aprepitant has been shown in animal models to inhibit emesis induced by cytotoxic chemotherapeutic agents, such as cisplatin, via central actions. Animal and human Positron Emission Tomography (PET) studies with aprepitant have shown that it crosses the blood brain barrier and occupies brain NK1 receptors. Animal and human studies show that aprepitant augments the antiemetic activity of the 5-HT3-receptor antagonist ondansetron and the corticosteroid dexamethasone and inhibits both the acute and delayed phases of cisplatin-induced emesis.
12.2 Pharmacodynamics
NK1 Receptor Occupancy
In two single-blind, multiple-dose, randomized, and placebo-controlled studies, healthy young men received oral EMEND doses of 10 mg (N=2), 30 mg (N=3), 100 mg (N=3) or 300 mg (N=5) once daily (0.08, 0.24, 0.8, and 2.4 times the maximum recommended single dose, respectively) for 14 days with 2 or 3 subjects on placebo. Both plasma aprepitant concentration and NK1 receptor occupancy in the corpus striatum by positron emission tomography were evaluated, at predose and 24 hours after the last dose. At aprepitant plasma concentrations of approximately 10 ng/mL and 100 ng/mL, the NK1 receptor occupancies were approximately 50% and 90%, respectively. The oral EMEND regimen produced mean trough plasma aprepitant concentrations greater than 500 ng/mL in adults, which would be expected to, based on the fitted curve with the Hill equation, result in greater than 95% brain NK1 receptor occupancy. However, the receptor occupancy has not been determined. In addition, the relationship between NK1 receptor occupancy and the clinical efficacy of EMEND has not been established.
Cardiac Electrophysiology
In a randomized, double-blind, positive-controlled, thorough QTc study, a single 200-mg dose of fosaprepitant had no effect on the QTc interval. Maximum aprepitant concentrations after a single 200-mg dose of fosaprepitant were 4-fold higher than that achieved with oral EMEND 125 mg. QT prolongation with the recommended oral EMEND dosing regimens is not expected.
12.3 Pharmacokinetics
Absorption
Following oral administration of a single 125-mg dose of EMEND on Day 1 and 80 mg once daily on Days 2 and 3, the AUC0-24hr was approximately 19.6 mcg∙hr/mL and 21.2 mcg∙hr/mL on Day 1 and Day 3, respectively. The Cmax of 1.6 mcg/mL and 1.4 mcg/mL were reached in approximately 4 hours (Tmax) on Day 1 and Day 3, respectively. At the dose range of 80 to 125 mg, the mean absolute oral bioavailability of EMEND is approximately 60 to 65%. Oral administration of the capsule with a standard high-fat breakfast had no clinically meaningful effect on the bioavailability of aprepitant.
The pharmacokinetics of aprepitant were non-linear across the clinical dose range. In healthy young adults, the increase in AUC0-∞ was 26% greater than dose proportional between 80-mg and 125-mg single doses administered in the fed state.
Distribution
Aprepitant is greater than 95% bound to plasma proteins. The mean apparent volume of distribution at steady state (Vdss) was approximately 70 L in humans.
Aprepitant crosses the blood brain barrier in humans [see Clinical Pharmacology (12.1)].
Elimination
Metabolism
Aprepitant undergoes extensive metabolism. In vitro studies using human liver microsomes indicate that aprepitant is metabolized primarily by CYP3A4 with minor metabolism by CYP1A2 and CYP2C19. Metabolism is largely via oxidation at the morpholine ring and its side chains. No metabolism by CYP2D6, CYP2C9, or CYP2E1 was detected. In healthy young adults, aprepitant accounts for approximately 24% of the radioactivity in plasma over 72 hours following a single oral 300-mg dose of [14C]-aprepitant (2.4 times the maximum EMEND recommended dose), indicating a substantial presence of metabolites in the plasma. Seven metabolites of aprepitant, which are only weakly active, have been identified in human plasma.
Excretion
Following administration of a single intravenous 100-mg dose of [14C]-aprepitant prodrug to healthy subjects, 57% of the radioactivity was recovered in urine and 45% in feces. A study was not conducted with radiolabeled capsule formulation. The results after oral administration may differ.
Aprepitant is eliminated primarily by metabolism; aprepitant is not renally excreted. The apparent plasma clearance of aprepitant ranged from approximately 62 to 90 mL/min. The apparent terminal half-life ranged from approximately 9 to 13 hours.
Specific Populations
Geriatric Patients
Following oral administration of a single 125-mg dose of EMEND on Day 1 and 80 mg once daily on Days 2 through 5 (2 additional days of dosing compared to the recommended duration), the AUC0-24hr of aprepitant was 21% higher on Day 1 and 36% higher on Day 5 in elderly (65 years and older) relative to younger adults. The Cmax was 10% higher on Day 1 and 24% higher on Day 5 in elderly relative to younger adults. These differences are not considered clinically meaningful [see Use in Specific Populations (8.5)].
Pediatric Patients
As part of a 3-day regimen, dosing of aprepitant capsules (125-mg/80-mg/80-mg) in 18 pediatric patients (aged 12 through 17 years) achieved a mean AUC0-24hr of 17 mcg∙hr/mL on Day 1 with mean peak plasma concentration (Cmax) at 1.3 mcg/mL occurring at approximately 4 hours. The mean concentrations at the end of Day 2 (N=8) and Day 3 (N=16) were both at 0.6 mcg/mL
As part of a 3-day regimen, weight-based dosing of aprepitant powder for oral suspension (3-mg/kg;2-mg/kg;2-mg/kg) in 18 pediatric patients aged 6 months to less than 12 years achieved a mean AUC0-24hr of 20.9 mcg∙hr/mL on Day 1 with mean peak plasma concentration (Cmax) at 1.8 mcg/mL (N=19), occurring at approximately 6 hours. The mean concentrations at the end of Day 2 (N=18) and Day 3 (N=19) were 0.4 mcg/mL and 0.5 mcg/mL, respectively [see Dosage and Administration (2.1)].
A population pharmacokinetic analysis of aprepitant in pediatric patients (aged 6 months through 17 years) suggests that sex and race have no clinically meaningful effect on the pharmacokinetics of aprepitant.
Male and Female Patients
Following oral administration of a single dose of aprepitant ranging from 40 mg to 375 mg (3 times the maximum EMEND recommended dose), the AUC0-24hr and Cmax are 9% and 17% higher in females as compared with males. The half-life of aprepitant is approximately 25% lower in females as compared with males and Tmax occurs at approximately the same time. These differences are not considered clinically meaningful.
Racial or Ethnic Groups
Following oral administration of a single dose of aprepitant ranging from 40 mg to 375 mg (3 times the maximum EMEND recommended dose), the AUC0-24hr and Cmax are approximately 27% and 19% higher in Hispanics as compared with Caucasians. The AUC0-24hr and Cmax were 74% and 47% higher in Asians as compared to Caucasians. There was no difference in AUC0-24hr or Cmax between Caucasians and Blacks. These differences are not considered clinically meaningful.
Patients with Renal Impairment
A single 240-mg dose of aprepitant (approximately 1.9 times the maximum EMEND recommended dose) was administered to patients with severe renal impairment (creatinine clearance less than 30 mL/min/1.73 m2 as measured by 24-hour urinary creatinine clearance) and to patients with end stage renal disease (ESRD) requiring hemodialysis.
In patients with severe renal impairment, the AUC0-∞ of total aprepitant (unbound and protein bound) decreased by 21% and Cmax decreased by 32%, relative to healthy subjects (creatinine clearance greater than 80 mL/min estimated by Cockcroft-Gault method). In patients with ESRD undergoing hemodialysis, the AUC0-∞ of total aprepitant decreased by 42% and Cmax decreased by 32%. Due to modest decreases in protein binding of aprepitant in patients with renal disease, the AUC of pharmacologically active unbound drug was not significantly affected in patients with renal impairment compared with healthy subjects. Hemodialysis conducted 4 or 48 hours after dosing had no significant effect on the pharmacokinetics of aprepitant; less than 0.2% of the dose was recovered in the dialysate [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Following administration of a single 125-mg dose of EMEND on Day 1 and 80 mg once daily on Days 2 and 3 to patients with mild hepatic impairment (Child-Pugh score 5 to 6), the AUC0-24hr of aprepitant was 11% lower on Day 1 and 36% lower on Day 3, as compared with healthy subjects given the same regimen. In patients with moderate hepatic impairment (Child-Pugh score 7 to 9), the AUC0-24hr of aprepitant was 10% higher on Day 1 and 18% higher on Day 3, as compared with healthy subjects given the same regimen. These differences in AUC0-24hr are not considered clinically meaningful. There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh score greater than 9) [see Use in Specific Populations (8.7)].
Drug Interactions Studies
Aprepitant is a substrate, a moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Aprepitant is also an inducer of CYP2C9. Aprepitant is unlikely to interact with drugs that are substrates for the P-glycoprotein transporter.
Effects of Aprepitant on the Pharmacokinetics of Other Drugs
CYP3A4 substrates (i.e., midazolam): Interactions between EMEND and coadministered midazolam are listed in Table 10 (increase is indicated as "↑", decrease as "↓", no change as "↔").
Table 10: Pharmacokinetic Interaction Data for EMEND and Coadministered Midazolam Dosage of EMEND Dosage of Midazolam Observed Drug Interactions EMEND 125 mg on Day 1 and 80 mg on Days 2 to 5 oral 2 mg single dose on Days 1 and 5 midazolam AUC ↑ 2.3-fold on Day 1 and ↑ 3.3-fold on Day 5 [see Drug Interactions (7.1)] EMEND 125 mg on Day 1 and 80 mg on Days 2 and 3 intravenous 2 mg prior to 3-day regimen of EMEND and on Days 4, 8 and 15 midazolam AUC ↑ 25% on Day 4, AUC ↓ 19% on Day 8 and AUC ↓ 4% on Day 15 EMEND 125 mg on Day 1 intravenous 2 mg given 1 hour after EMEND midazolam AUC ↑ 1.5-fold A difference of less than 2-fold increase of midazolam AUC is not considered clinically important.
Corticosteroids:
Dexamethasone: EMEND, when given as a regimen of 125 mg on Day 1 and 80 mg/day on Days 2 through 5, coadministered with 20-mg dexamethasone on Day 1 and 8-mg dexamethasone on Days 2 through 5, increased the AUC of dexamethasone by 2.2-fold on Days 1 and 5 [see Dosage and Administration (2.1)].
Chemotherapeutic agents:
CYP2C9 substrates (Warfarin, Tolbutamide):
Warfarin: A single 125-mg dose of EMEND was administered on Day 1 and 80 mg/day on Days 2 and 3 to healthy subjects who were stabilized on chronic warfarin therapy. Although there was no effect of EMEND on the plasma AUC of R(+) or S(-) warfarin determined on Day 3, there was a 34% decrease in S(-) warfarin trough concentration accompanied by a 14% decrease in the prothrombin time (reported as International Normalized Ratio or INR) 5 days after completion of dosing with EMEND [see Drug Interactions (7.1)].
Tolbutamide: EMEND, when given as 125 mg on Day 1 and 80 mg/day on Days 2 and 3, decreased the AUC of tolbutamide by 23% on Day 4, 28% on Day 8, and 15% on Day 15, when a single dose of tolbutamide 500 mg was administered prior to the administration of the 3-day regimen of EMEND and on Days 4, 8, and 15. This effect was not considered clinically important.
Other Drugs
Oral contraceptives: When EMEND was administered as a 3-day regimen (125-mg/80-mg/80-mg) with ondansetron and dexamethasone, and coadministered with an oral contraceptive containing ethinyl estradiol and norethindrone, the trough concentrations of both ethinyl estradiol and norethindrone were reduced by as much as 64% for 3 weeks post-treatment.
Effect of Other Drugs on the Pharmacokinetics of Aprepitant
Ketoconazole: When a single 125-mg dose of EMEND was administered on Day 5 of a 10-day regimen of 400 mg/day of ketoconazole, a strong CYP3A4 inhibitor, the AUC of aprepitant increased approximately 5-fold and the mean terminal half-life of aprepitant increased approximately 3-fold [see Drug Interactions (7.2)].
Rifampin: When a single 375-mg dose of aprepitant (3 times the maximum EMEND recommended dose) was administered on Day 9 of a 14-day regimen of 600 mg/day of rifampin, a strong CYP3A4 inducer, the AUC of aprepitant decreased approximately 11-fold and the mean terminal half-life decreased approximately 3-fold [see Drug Interactions (7.2)].
Diltiazem: In patients with mild to moderate hypertension, administration of aprepitant once daily, as a tablet formulation comparable to 230 mg of the capsule formulation (approximately 1.8 times the EMEND recommended dose), with diltiazem 120 mg 3 times daily for 5 days, resulted in a 2-fold increase of aprepitant AUC and a simultaneous 1.7-fold increase of diltiazem AUC. These pharmacokinetic effects did not result in clinically meaningful changes in ECG, heart rate or blood pressure beyond those changes induced by diltiazem alone [see Drug Interactions (7.2)].
Paroxetine: Coadministration of once daily doses of aprepitant, as a tablet formulation comparable to 85 mg or 170 mg of the capsule formulation (approximately 0.7 and 1.4 times the maximum EMEND recommended dose), with paroxetine 20 mg once daily, resulted in a decrease in AUC by approximately 25% and Cmax by approximately 20% of both aprepitant and paroxetine. This effect was not considered clinically important.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies were conducted in Sprague-Dawley rats and in CD-1 mice for 2 years. In the rat carcinogenicity studies, animals were treated with oral doses ranging from 0.05 to 1000 mg/kg twice daily. The highest dose produced a systemic exposure to aprepitant (AUC) of 0.7 to 1.6 times the adult human exposure at the 125-mg/80-mg/80-mg EMEND regimen. Treatment with aprepitant at doses of 5 to 1000 mg/kg twice daily caused an increase in the incidences of thyroid follicular cell adenomas and carcinomas in male rats. In female rats, it produced hepatocellular adenomas at 5 to 1000 mg/kg twice daily and hepatocellular carcinomas and thyroid follicular cell adenomas at 125 to 1000 mg/kg twice daily. In the mouse carcinogenicity studies, the animals were treated with oral doses ranging from 2.5 to 2000 mg/kg/day. The highest dose produced a systemic exposure of about 2.8 to 3.6 times the adult human exposure at the 125-mg/80-mg/80-mg EMEND regimen. Treatment with aprepitant produced skin fibrosarcomas at 125 and 500 mg/kg/day doses in male mice.
Mutagenesis
Aprepitant was not genotoxic in the Ames test, the human lymphoblastoid cell (TK6) mutagenesis test, the rat hepatocyte DNA strand break test, the Chinese hamster ovary (CHO) cell chromosome aberration test and the mouse micronucleus test.
Impairment of Fertility
Aprepitant did not affect the fertility or general reproductive performance of male or female rats at doses up to the maximum feasible dose of 1000 mg/kg twice daily (providing exposure in male rats lower than the exposure at the recommended adult human dose and exposure in female rats at about 1.6 times the adult human exposure at the 125-mg/80-mg/80-mg EMEND regimen).
-
14 CLINICAL STUDIES
14.1 Prevention of Nausea and Vomiting Associated with HEC in Adults
Oral administration of EMEND in combination with ondansetron and dexamethasone (EMEND regimen) has been shown to prevent acute and delayed nausea and vomiting associated with HEC including high-dose cisplatin, and nausea and vomiting associated with MEC.
In Studies 1 and 2, both multicenter, randomized, parallel, double-blind, controlled clinical studies in adults, EMEND in combination with ondansetron and dexamethasone was compared with standard therapy (ondansetron and dexamethasone alone) in patients receiving a chemotherapy regimen that included cisplatin greater than 50 mg/m2 (mean cisplatin dose = 80.2 mg/m2). See Table 11.
In these studies, 95% of the patients in the EMEND group received a concomitant chemotherapeutic agent in addition to protocol-mandated cisplatin. The most common chemotherapeutic agents and the number of EMEND patients exposed follows: etoposide (106), fluorouracil (100), gemcitabine (89), vinorelbine (82), paclitaxel (52), cyclophosphamide (50), doxorubicin (38), docetaxel (11).
Of the 550 patients who were randomized to receive the EMEND regimen, 42% were women, 58% men, 59% White, 3% Asian, 5% Black, 12% Hispanic American, and 21% Multi-Racial. The EMEND-treated patients in these clinical studies ranged from 14 to 84 years of age, with a mean age of 56 years. A total of 170 patients were 65 years or older, with 29 patients being 75 years or older.
Table 11: HEC Treatment Regimens – Studies 1 and 2* Day 1 Day 2 Day 3 Day 4 - * EMEND placebo and dexamethasone placebo were used to maintain blinding.
- † EMEND was administered 1 hour prior to chemotherapy treatment on Day 1 and in the morning on Days 2 and 3.
- ‡ Dexamethasone was administered 30 minutes prior to chemotherapy treatment on Day 1 and in the morning on Days 2 through 4. The 12 mg dose of dexamethasone on Day 1 reflects a dosage adjustment to account for a drug interaction with the EMEND regimen [see Clinical Pharmacology (12.3)].
- § Ondansetron 32 mg intravenous was used in the clinical trials of EMEND. Although this dose was used in clinical trials, this is no longer the currently recommended dose. Refer to the ondansetron prescribing information for the current recommended dose.
CINV EMEND Regimen Oral EMEND† 125 mg 80 mg 80 mg none Oral Dexamethasone‡ 12 mg 8 mg 8 mg 8 mg Ondansetron 5-HT3 antagonist§ none none none CINV Standard Therapy Oral Dexamethasone 20 mg 8 mg twice daily 8 mg twice daily 8 mg twice daily Ondansetron 5-HT3 antagonist§ none none none The antiemetic activity of EMEND was evaluated during the acute phase (0 to 24 hours post-cisplatin treatment), the delayed phase (25 to 120 hours post-cisplatin treatment) and overall (0 to 120 hours post-cisplatin treatment) in Cycle 1. Efficacy was based on evaluation of the following endpoints in which emetic episodes included vomiting, retching, or dry heaves:
Primary endpoint:
- complete response (defined as no emetic episodes and no use of rescue therapy as recorded in patient diaries)
Other prespecified endpoints:
- complete protection (defined as no emetic episodes, no use of rescue therapy, and a maximum nausea visual analogue scale [VAS] score less than 25 mm on a 0 to 100 mm scale)
- no emesis (defined as no emetic episodes regardless of use of rescue therapy)
- no nausea (maximum VAS less than 5 mm on a 0 to 100 mm scale)
- no significant nausea (maximum VAS less than 25 mm on a 0 to 100 mm scale)
A summary of the key study results from each individual study analysis is shown in Table 12. In both studies, a statistically significantly higher proportion of patients receiving the EMEND regimen in Cycle 1 had a complete response in the overall phase (primary endpoint), compared with patients receiving standard therapy. A statistically significant difference in complete response in favor of the EMEND regimen was also observed when the acute phase and the delayed phase were analyzed separately.
Table 12: Percent of Patients Receiving HEC Responding by Treatment Group and Phase — Cycle 1 Study 1 Study 2 ENDPOINTS EMEND Regimen
(N=260)*
%Standard Therapy
(N=261)*
%p-Value EMEND Regimen
(N=261)*
%Standard Therapy
(N=263)*
%p-Value Visual analogue scale (VAS) score range: 0 mm=no nausea; 100 mm=nausea as bad as it could be. - * N: Number of patients (older than 18 years of age) who received cisplatin, study drug, and had at least one post-treatment efficacy evaluation.
- † Overall: 0 to 120 hours post-cisplatin treatment.
- ‡ Acute phase: 0 to 24 hours post-cisplatin treatment.
- § Delayed phase: 25 to 120 hours post-cisplatin treatment.
- ¶ Not statistically significant when adjusted for multiple comparisons.
- # Not statistically significant.
PRIMARY ENDPOINT Complete Response Overall† 73 52 <0.001 63 43 <0.001 OTHER PRESPECIFIED ENDPOINTS Complete Response Acute phase‡ 89 78 <0.001 83 68 <0.001 Delayed phase§ 75 56 <0.001 68 47 <0.001 Complete Protection Overall 63 49 0.001 56 41 <0.001 Acute phase 85 75 NS¶ 80 65 <0.001 Delayed phase 66 52 <0.001 61 44 <0.001 No Emesis Overall 78 55 <0.001 66 44 <0.001 Acute phase 90 79 0.001 84 69 <0.001 Delayed phase 81 59 <0.001 72 48 <0.001 No Nausea Overall 48 44 NS# 49 39 NS¶ Delayed phase 51 48 NS# 53 40 NS¶ No Significant Nausea Overall 73 66 NS# 71 64 NS# Delayed phase 75 69 NS# 73 65 NS# In both studies, the estimated time to first emesis after initiation of cisplatin treatment was longer with the EMEND regimen, and the incidence of first emesis was reduced in the EMEND regimen group compared with standard therapy group as depicted in the Kaplan-Meier curves in Figure 1.
Figure 1: Percent of Patients Receiving HEC Who Remain Emesis Free Over Time — Cycle 1 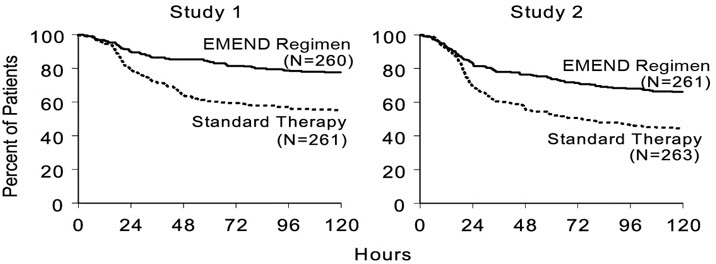
p-Value <0.001 based on a log rank test for Study 1 and Study 2; nominal p-values not adjusted for multiplicity. Additional Patient-Reported Outcomes: The impact of nausea and vomiting on patients' daily lives was assessed in Cycle 1 of both studies using the Functional Living Index–Emesis (FLIE), a validated nausea- and vomiting-specific patient-reported outcome measure. Minimal or no impact of nausea and vomiting on patients' daily lives is defined as a FLIE total score greater than 108. In each of the 2 studies, a higher proportion of patients receiving the EMEND regimen reported minimal or no impact of nausea and vomiting on daily life (Study 1: 74% versus 64%; Study 2: 75% versus 64%).
Multiple-Cycle Extension: In the same 2 clinical studies, patients continued into the Multiple-Cycle extension for up to 5 additional cycles of chemotherapy. The proportion of patients with no emesis and no significant nausea by treatment group at each cycle is depicted in Figure 2. Antiemetic effectiveness for the patients receiving the EMEND regimen was maintained throughout repeat cycles for those patients continuing in each of the multiple cycles.
Figure 2: Proportion of Patients Receiving HEC with No Emesis and No Significant Nausea by Treatment Group and Cycle 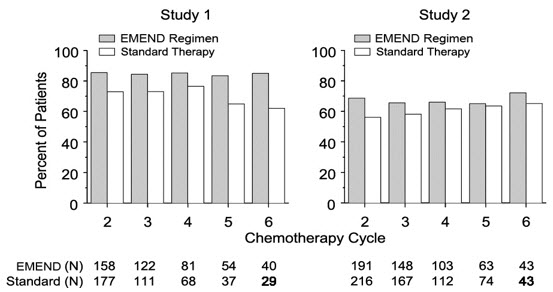
14.2 Prevention of Nausea and Vomiting Associated with MEC in Adults
EMEND was studied in two randomized, double-blind, parallel-group studies (Studies 3 and 4) in adult patients receiving MEC.
In Study 3, in breast cancer patients, EMEND in combination with ondansetron and dexamethasone was compared with standard therapy (ondansetron and dexamethasone) in patients receiving a MEC regimen that included cyclophosphamide 750-1500 mg/m2; or cyclophosphamide 500-1500 mg/m2 and doxorubicin (less than or equal to 60 mg/m2) or epirubicin (less than or equal to 100 mg/m2). See Table 13.
In this study, the most common combinations were cyclophosphamide + doxorubicin (61%); and cyclophosphamide + epirubicin + fluorouracil (22%).
Of the 438 patients who were randomized to receive the EMEND regimen, 99.5% were women. Of these, approximately 80% were White, 8% Black, 8% Asian, 4% Hispanic, and less than 1% Other. The EMEND-treated patients in this clinical study ranged from 25 to 78 years of age, with a mean age of 53 years; 70 patients were 65 years or older, with 12 patients being over 74 years.
Table 13: MEC Treatment Regimens – Studies 3 and 4* Day 1 Day 2 Day 3 - * EMEND placebo and dexamethasone placebo were used to maintain blinding.
- † EMEND was administered 1 hour prior to chemotherapy treatment on Day 1 and in the mornings on Days 2 and 3.
- ‡ Dexamethasone was administered 30 minutes prior to chemotherapy treatment on Day 1. The 12 mg dose of dexamethasone on Day 1 reflects a dosage adjustment to account for a drug interaction with the EMEND regimen [see Clinical Pharmacology (12.3)].
- § The first ondansetron dose was administered 30 to 60 minutes prior to chemotherapy treatment on Day 1 and the second dose was administered 8 hours after first ondansetron dose.
CINV EMEND Regimen Oral EMEND† 125 mg 80 mg 80 mg Oral Dexamethasone 12 mg‡ none none Oral Ondansetron 8 mg × 2 doses§ none none CINV Standard Therapy Oral Dexamethasone 20 mg‡ none none Oral Ondansetron 8 mg × 2 doses§ 8 mg twice daily 8 mg twice daily The antiemetic activity of EMEND was evaluated based on the following endpoints in which emetic episodes included vomiting, retching, or dry heaves:
Primary endpoint:
- complete response (defined as no emetic episodes and no use of rescue therapy as recorded in patient diaries) in the overall phase (0 to 120 hours post-chemotherapy)
Other prespecified endpoints:
- no emesis (defined as no emetic episodes regardless of use of rescue therapy)
- no nausea (maximum VAS less than 5 mm on a 0 to 100 mm scale)
- no significant nausea (maximum VAS less than 25 mm on a 0 to 100 mm scale)
- complete protection (defined as no emetic episodes, no use of rescue therapy, and a maximum nausea visual analogue scale [VAS] score less than 25 mm on a 0 to 100 mm scale)
- complete response during the acute and delayed phases.
A summary of the key results from Study 3 is shown in Table 14. In Study 3, a statistically significantly (p=0.015) higher proportion of patients receiving the EMEND regimen (51%) in Cycle 1 had a complete response (primary endpoint) during the overall phase compared with patients receiving standard therapy (42%). The difference between treatment groups was primarily driven by the "No Emesis Endpoint", a principal component of this composite primary endpoint. In addition, a higher proportion of patients receiving the EMEND regimen in Cycle 1 had a complete response during the acute (0-24 hours) and delayed (25-120 hours) phases compared with patients receiving standard therapy; however, the treatment group differences failed to reach statistical significance, after multiplicity adjustments.
Table 14: Percent of Patients Receiving MEC Responding by Treatment Group and Phase — Cycle 1 of Study 3 ENDPOINTS EMEND Regimen
(N=433)*
%Standard Therapy
(N=424)*
%p-Value - * N: Number of patients included in the primary analysis of complete response.
- † Overall: 0 to 120 hours post-chemotherapy treatment.
- ‡ NS when adjusted for prespecified multiple comparisons rule; unadjusted p-value <0.001.
PRIMARY ENDPOINT† Complete Response 51 42 0.015 OTHER PRESPECIFIED ENDPOINTS† No Emesis 76 59 NS‡ No Nausea 33 33 NS No Significant Nausea 61 56 NS No Rescue Therapy 59 56 NS Complete Protection 43 37 NS Additional Patient-Reported Outcomes: In Study 3, in patients receiving MEC, the impact of nausea and vomiting on patients' daily lives was assessed in Cycle 1 using the FLIE. A higher proportion of patients receiving the EMEND regimen reported minimal or no impact on daily life (64% versus 56%). This difference between treatment groups was primarily driven by the "No Vomiting Domain" of this composite endpoint.
Multiple-Cycle Extension: In Study 3, patients receiving MEC were permitted to continue into the Multiple-Cycle extension of the study for up to 3 additional cycles of chemotherapy. The antiemetic effect for patients receiving the EMEND regimen was maintained during all cycles.
In Study 4, EMEND in combination with ondansetron and dexamethasone was compared with a standard therapy (ondansetron and dexamethasone alone) in patients receiving a MEC regimen that included any intravenous dose of oxaliplatin, carboplatin, epirubicin, idarubicin, ifosfamide, irinotecan, daunorubicin, doxorubicin; cyclophosphamide intravenous (less than 1500 mg/m2); or cytarabine intravenous (greater than 1 g/m2). See Table 13. Patients receiving the EMEND regimen were receiving chemotherapy for a variety of tumor types including 50% with breast cancer, 21% with gastrointestinal cancers including colorectal cancer, 13% with lung cancer and 6% with gynecological cancers.
Of the 430 patients who were randomized to receive the EMEND regimen, 76% were women and 24% were men. The distribution by race was 67% White, 6% Black or African American, 11% Asian, and 12% multiracial. Classified by ethnicity, 36% were Hispanic and 64% were non-Hispanic. The EMEND-treated patients in this clinical study ranged from 22 to 85 years of age, with a mean age of 57 years; approximately 59% of the patients were 55 years or older with 32 patients being over 74 years.
The antiemetic activity of EMEND was evaluated based on no vomiting (with or without rescue therapy) in the overall period (0 to 120 hours post-chemotherapy) and complete response (defined as no vomiting and no use of rescue therapy) in the overall period.
A summary of the key results from Study 4 is shown in Table 15. In Study 4, a statistically significantly higher proportion of patients receiving the EMEND regimen (76%) in Cycle 1 had no vomiting during the overall phase compared with patients receiving standard therapy (62%). In addition, a higher proportion of patients receiving the EMEND regimen (69%) in Cycle 1 had a complete response in the overall phase (0-120 hours) compared with patients receiving standard therapy (56%). In the acute phase (0 to 24 hours following initiation of chemotherapy), a higher proportion of patients receiving EMEND compared to patients receiving standard therapy were observed to have no vomiting (92% and 84%, respectively) and complete response (89% and 80%, respectively). In the delayed phase (25 to 120 hours following initiation of chemotherapy), a higher proportion of patients receiving EMEND compared to patients receiving standard therapy were observed to have no vomiting (78% and 67%, respectively) and complete response (71% and 61%, respectively).
In a subgroup analysis by tumor type, a numerically higher proportion of patients receiving EMEND were observed to have no vomiting and complete response compared to patients receiving standard therapy. For sex, the difference in complete response rates between the EMEND and standard regimen groups was 14% in females (64.5% and 50.3%, respectively) and 4% in males (82.2% and 78.2%, respectively) during the overall phase. A similar difference for sex was observed for the no vomiting endpoint.
Table 15: Percent of Patients Receiving MEC Responding by Treatment Group — Cycle 1 of Study 4 ENDPOINTS EMEND Regimen
(N=430)*
%Standard Therapy
(N=418)*
%p-Value - * N = Number of patients who received chemotherapy treatment, study drug, and had at least one post-treatment efficacy evaluation.
No Vomiting Overall 76 62 <0.0001 Complete Response Overall 69 56 0.0003 14.3 Prevention of Nausea and Vomiting Associated with HEC or MEC in Pediatric Patients
In a randomized, double-blind, active comparator-controlled clinical study that included 302 pediatric patients aged 6 months to 17 years receiving HEC or MEC, EMEND in combination with ondansetron was compared to ondansetron alone (control regimen) for the prevention of CINV (Study 5). Intravenous dexamethasone was permitted as part of the antiemetic regimen in both treatment groups, at the discretion of the physician. A 50% dose reduction of dexamethasone was required for patients in the EMEND group, reflecting a dosage adjustment to account for a drug interaction [see Clinical Pharmacology (12.3)]. No dexamethasone dose reduction was required for patients who received the control regimen.
Eligible patients had documented malignancy at either an original diagnosis or relapse and were scheduled to receive emetogenic chemotherapy or a chemotherapy regimen not previously tolerated due to vomiting along with ondansetron as part of their antiemetic regimen.
Of the 152 pediatric patients randomized to receive the EMEND regimen, 55% were male, 45% female, 78% White, 7% Asian, 0% Black, 24% Hispanic, and 13% Multi-Racial. The most common primary malignancies in subjects receiving the EMEND regimen were osteosarcoma (11%), Ewing's sarcoma (11%), neuroblastoma (9%) and rhabdomyosarcoma (8%). Other concomitant chemotherapy agents commonly administered and the number of EMEND patients exposed were: vincristine sulfate (65), etoposide (59), doxorubicin (48), ifosfamide (45), carboplatin (39), and cisplatin (35).
The treatment regimens in Study 5 for pediatric patients are defined in Table 16. Of the pediatric patients, 29% in the EMEND regimen and 28% in the control regimen used dexamethasone as part of the antiemetic regimen in Cycle 1.
Table 16: HEC and MEC Treatment Regimens* for Pediatric Patients 6 Months to 17 Years of Age— Study 5 Day 1 Day 2 Day 3 - * Intravenous dexamethasone was permitted at the discretion of the physician. A 50% dose reduction of dexamethasone was required for patients in the EMEND group, reflecting a dosage adjustment to account for a drug interaction [see Clinical Pharmacology (12.3)]. No dexamethasone dose reduction was required for patients in the control regimen.
- † EMEND was administered 1 hour prior to chemotherapy treatment on Days 1, 2, and 3. If no chemotherapy was given on Days 2 and 3, EMEND was administered in the morning.
- ‡ Ondansetron was administered 30 minutes prior to chemotherapy on Day 1
- § EMEND placebo was used to maintain blinding.
CINV EMEND Regimen Pediatric Patients 6 Months to less than 12 Years of Age† 3 mg/kg body weight oral suspension 2 mg/kg body weight oral suspension 2 mg/kg body weight oral suspension Pediatric Patients 12 to 17 Years of Age† 125 mg capsule 80 mg capsule 80 mg capsule Ondansetron Per standard of care‡ none none CINV Control Regimen§ Ondansetron Per standard of care‡ none none The antiemetic activity of EMEND was evaluated over a 5-day (120 hour) period following the initiation of chemotherapy on Day 1. The primary endpoint in Study 5 was complete response in the delayed phase (25 to 120 hours following chemotherapy) in Cycle 1. Patients had the opportunity to receive open-label EMEND in subsequent cycles (Optional Cycles 2-6); however, efficacy was not assessed in these optional cycles. Overall efficacy was based on the evaluation of the following endpoints:
Primary endpoint:
- complete response (no vomiting, retching and no use of rescue medication) in the delayed phase (25 to 120 hours following initiation of chemotherapy)
Other prespecified endpoints:
- complete response in the acute phase (0 to 24 hours following initiation of chemotherapy)
- complete response in the overall phase (up to 120 hours following initiation of chemotherapy)
- no vomiting (defined as no emesis, retching or dry heaves, regardless of use of rescue medication) in the overall phase
- safety and tolerability
A summary of the key study results is shown in Table 17.
Table 17: Percent of Patients Who Responded to Treatment by Treatment Group and Phase – Cycle 1 of Study 5 EMEND Regimen
n/m (%)Control Regimen
n/m (%)n/m = Number of patients with desired response/number of patients included in time point. Acute Phase: 0 to 24 hours following initiation of chemotherapy. Delayed Phase: 25 to 120 hours following initiation of chemotherapy. Overall Phase: 0 to 120 hours following initiation of chemotherapy. - * Complete Response = No vomiting or retching and no use of rescue medication.
- † p<0.01 when compared to Control Regimen
- ‡ p<0.05 when compared to Control Regimen
PRIMARY ENDPOINT Complete Response* - Delayed phase 77/152 (50.7)† 39/150 (26.0) OTHER PRESPECIFIED ENDPOINTS Complete Response* – Acute phase 101/152 (66.4)‡ 78/150 (52.0) Complete Response* – Overall phase 61/152 (40.1)† 30/150 (20.0) -
16 HOW SUPPLIED/STORAGE AND HANDLING
No. 3855 — 125-mg capsules: Opaque, hard gelatin capsule with white body and pink cap with "462" and "125 mg" printed radially in black ink on the body. They are supplied as follows:
NDC: 0006-0462-06 unit-dose package of 6.
No. 3854 — 80-mg capsules: White, opaque, hard gelatin capsule with "461" and "80 mg" printed radially in black ink on the body. They are supplied as follows:
NDC: 0006-0461-02 unit-of-use BiPack of 2
NDC: 0006-0461-06 unit-dose package of 6.
No. 3862 — Unit-of-use TriPack containing one 125-mg capsule and two 80-mg capsules.
NDC: 0006-3862-03.
No. 3066 — 125 mg for oral suspension: Pink to light pink powder, in a single-use pouch, packaged as a kit with one 1 mL oral dosing dispenser, one 5 mL oral dosing dispenser, one cap and one mixing cup. It is supplied as follows:
NDC: 0006-3066-03 – unit of use carton.
Storage and Handling
For Oral Suspension
Store unopened pouch at 20-25°C (68-77°F); excursions permitted between 15-30°C (between 59-86°F). Store in the original container. Do not open pouch until ready for use.
Once prepared, if suspension is not used immediately, store refrigerated [between 36°F-46°F (2°C-8°C)] for up to 72 hours prior to use. When ready to use, the mixture can be kept at room temperature [between 68°F-77°F (20°C-25°C)] for up to 3 hours.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions, including anaphylaxis, have been reported in patients taking EMEND. Advise patients to stop taking EMEND and seek immediate medical attention if they experience signs or symptoms of a hypersensitivity reaction, such as hives, rash and itching, skin peeling or sores, or difficulty in breathing or swallowing.
Drug Interactions
Advise patients to discuss all medications they are taking, including other prescription, non-prescription medication or herbal products [see Contraindications (4), Warnings and Precautions (5.1)].
Warfarin: Instruct patients on chronic warfarin therapy to follow instructions from their healthcare provider regarding blood draws to monitor their INR during the 2-week period, particularly at 7 to 10 days, following initiation of the 3-day regimen of EMEND with each chemotherapy cycle [see Warnings and Precautions (5.2)].
Hormonal Contraceptives: Advise patients that administration of EMEND may reduce the efficacy of hormonal contraceptives. Instruct patients to use effective alternative or back-up methods of contraception (such as condoms and spermicides) during treatment with EMEND and for 1 month following the last dose of EMEND [see Warnings and Precautions (5.3), Use in Specific Populations (8.3)].
-
SPL UNCLASSIFIED SECTION
Distributed by:
Merck Sharp & Dohme Corp., a subsidiary of MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
Copyright © 2003-2019 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
uspi-mk0869-mf-1911r014
-
PATIENT PACKAGE INSERT
Patient Information
EMEND® (EE mend)
(aprepitant)
capsules
EMEND® (EE mend)
(aprepitant)
for oral suspensionThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: September 2019 Read this Patient Information before you start taking EMEND and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment. What is EMEND? EMEND for oral suspension is a prescription medicine used: - with other medicines that treat nausea and vomiting in patients 6 months of age and older to prevent nausea and vomiting caused by certain anti-cancer (chemotherapy) medicines
EMEND capsules is a prescription medicine used: - with other medicines that treat nausea and vomiting in patients 12 years of age and older to prevent nausea and vomiting caused by certain anti-cancer (chemotherapy) medicines
EMEND is not used to treat nausea and vomiting that you already have. EMEND should not be used continuously for a long time (chronic use). Who should not take EMEND? Do not take EMEND if you: - are allergic to aprepitant or any of the ingredients in EMEND. See the end of this leaflet for a complete list of ingredients in EMEND.
- are taking pimozide (ORAP®)
What should I tell my healthcare provider before taking EMEND? Before you take EMEND, tell your healthcare provider if you: - have liver problems
- are pregnant or plan to become pregnant. It is not known if EMEND can harm your unborn baby.
- Women who use birth control medicines containing hormones to prevent pregnancy (birth control pills, skin patches, implants, and certain IUDs) should also use a back-up method of birth control that does not contain hormones, such as condoms and spermicides, during treatment with EMEND and for 1 month after your last dose of EMEND.
- are breastfeeding or plan to breastfeed. It is not known if EMEND passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take EMEND.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. EMEND may affect the way other medicines work, and other medicines may affect how EMEND works causing serious side effects. Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine. How should I take EMEND? - Take EMEND exactly as prescribed.
- Swallow EMEND capsules whole.
- EMEND may be taken with or without food.
- If you take too much EMEND, call your healthcare provider, or go to the nearest hospital emergency room.
- EMEND is taken as 3 doses over 3 days - starting on the day you have chemotherapy, and for the following 2 days.
-
In adults, there are 2 ways your healthcare provider may prescribe EMEND for you:
- 1.
Capsules of EMEND by mouth for all 3 doses:
- You should get a package that has 3 capsules of EMEND.
- Day 1 (Day of chemotherapy): Take one 125 mg capsule of EMEND (white and pink) by mouth 1 hour before you start your chemotherapy treatment.
- Day 2 and Day 3: Take one 80 mg capsule of EMEND (white) by mouth 1 hour before you start your chemotherapy treatment. If no chemotherapy treatment is given on Days 2 and 3, EMEND should be taken in the morning.
- 1.
Capsules of EMEND by mouth for all 3 doses:
- 2.
Oral suspension of EMEND by mouth for all 3 doses, for adults who are not able to swallow capsules:
- For each dose of EMEND for oral suspension, you will get a pre-filled oral dosing dispenser that contains your prescribed dose.
- See the detailed Instructions for Use that comes with EMEND for oral suspension for information about the correct way to take a dose of EMEND for oral suspension. If you have questions about how to take EMEND for oral suspension, talk to your healthcare provider.
- Day 1 (Day of chemotherapy): Take 1 dose of EMEND for oral suspension by mouth 1 hour before you start your chemotherapy treatment.
- Day 2 and Day 3: Take 1 dose of EMEND for oral suspension by mouth 1 hour before you start your chemotherapy treatment. If no chemotherapy treatment is given on Days 2 and 3, EMEND for oral suspension should be taken in the morning.
- In children 12 years of age and older who can swallow capsules by mouth, EMEND is prescribed as capsules of EMEND by mouth for all 3 doses:
- You should get a package that has 3 capsules of EMEND.
- Day 1 (Day of chemotherapy): Take one 125 mg capsule of EMEND (white and pink) by mouth 1 hour before you start your chemotherapy treatment.
- Day 2 and Day 3: Take one 80 mg capsule of EMEND (white) by mouth 1 hour before you start your chemotherapy treatment. If no chemotherapy treatment is given on Days 2 and 3, EMEND should be taken in the morning.
- In children 6 months to less than 12 years of age or in children 12 years of age and older who are not able to swallow capsules, EMEND is prescribed as oral suspension of EMEND by mouth for all 3 doses:
- For each dose of EMEND, you will get a pre-filled oral dosing dispenser that contains your child's prescribed dose.
- See the detailed Instructions for Use that comes with EMEND for oral suspension, for information about the correct way to give a dose of EMEND for oral suspension. If you have questions about how to give EMEND for oral suspension, talk to your child's healthcare provider.
- Day 1 (Day of chemotherapy): Give 1 dose of EMEND for oral suspension to your child by mouth 1 hour before they start their chemotherapy treatment.
- Day 2 and Day 3: Give 1 dose of EMEND for oral suspension to your child by mouth 1 hour before they start their chemotherapy treatment. If no chemotherapy treatment is given on Days 2 and 3, EMEND should be given in the morning.
- If you take the blood thinner medicine warfarin sodium (COUMADIN®, JANTOVEN®), your healthcare provider may do blood tests after you take EMEND to check your blood clotting.
What are the possible side effects of EMEND? - In adults taking EMEND, the most common side effects include tiredness, diarrhea, weakness, indigestion, stomach (abdominal) pain, hiccups, decrease in white blood cell count, dehydration, and changes in liver function tests.
- In children 6 months to 17 years of age, the most common side effects include decrease in white blood cell count, headache, diarrhea, decreased appetite, cough, tiredness, decrease in red blood cell count, dizziness, and hiccups.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of EMEND. For more information ask your healthcare provider or pharmacist. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store EMEND? EMEND capsules - Store EMEND capsules at room temperature, between 68°F to 77°F (20°C to 25°C).
EMEND for oral suspension - Store EMEND for oral suspension in the refrigerator, between 36°F to 46°F (2°C to 8°C).
- Use EMEND for oral suspension within 2 days of getting the medicine from your healthcare provider.
- When ready to use, EMEND for oral suspension can be kept at room temperature, between 68°F to 77°F (20°C to 25°C) for up to 3 hours.
Keep EMEND and all medicines out of the reach of children. General information about the safe and effective use of EMEND Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use EMEND for a condition for which it was not prescribed. Do not give EMEND to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about EMEND that is written for health professionals. For more information about EMEND call 1-800-622-4477 or go to www.emend.com. What are the ingredients in EMEND? EMEND capsules: Active ingredient: aprepitant Inactive ingredients: sucrose, microcrystalline cellulose, hydroxypropyl cellulose and sodium lauryl sulfate. The capsule shell excipients are gelatin, titanium dioxide, and may contain sodium lauryl sulfate and silicon dioxide. The 125 mg capsule shell also contains red ferric oxide and yellow ferric oxide. EMEND for oral suspension: Active ingredient: aprepitant Inactive ingredients: sucrose, lactose, hydroxypropyl cellulose, sodium lauryl sulfate, red iron oxide, and sodium stearyl fumarate. Distributed by: Merck Sharp & Dohme Corp., a subsidiary of MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
The brands listed in the above sections "Who should not take EMEND?" and "How should I take EMEND?" are the registered trademarks of their respective owners and are not trademarks of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
Copyright © 2003-2019 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
usppi-mk0869-mf-1909r007 -
INSTRUCTIONS FOR USE
Instructions for Use EMEND® (EE-mend)
(aprepitant)
for oral suspension Read the Patient Information and Instructions for
Read the Patient Information and Instructions for
Use for EMEND for oral suspension before you take or
before you give a dose to your childTake by mouth only
How to give a dose of EMEND for oral suspension The healthcare provider has prepared the dose of EMEND for you or your child. - 1. You will get EMEND in an oral dosing dispenser
- 2. Keep the oral dosing dispenser in the refrigerator until you give EMEND to yourself or your child

- Store EMEND in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Use EMEND within 2 days of getting the medicine from the healthcare provider.
- When ready to use, EMEND can be kept at room temperature, between 68°F to 77°F (20°C to 25°C) for up to 3 hours.
- Keep EMEND and all medicines out of the reach of children.
- 3. Give EMEND
- 4. Throw away the oral dosing dispenser and cap

The color of the medicine in the oral dosing dispenser may be different shades of pink (light pink to dark pink). This is normal and the medicine is okay to use. - Take the cap off the oral dosing dispenser.
- Place the tip of the oral dosing dispenser in your mouth or in your child's mouth along the inner cheek on either the right or left side.
- Slowly push the plunger all the way down to give all of the medicine in the oral dosing dispenser.
Call the healthcare provider if you or your child is not able to take the prescribed dose.
For more information go to www.emend.com or call 1-800-622-4477.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Merck Sharp & Dohme Corp., a subsidiary of MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
Copyright © 2015 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
Issued: 12/2015
usifu-mk0869-mf-1512r000 -
PRINCIPAL DISPLAY PANEL - 80 mg Capsule Dose Pack
NDC: 0006-0461-02
BiPack
2 capsulesEMEND®
(aprepitant) capsules80mg
For Adults and
Pediatric Patients
12 years of Age and OlderYou have already been given
a capsule of EMEND (aprepitant) to
prevent nausea and vomiting before
your chemotherapy on Day 1.The two capsules in this package
are to be taken on Days 2 and 3.Each capsule contains 80 mg
of aprepitant.USUAL DOSAGE:
See accompanying circular.Rx only
Keep this and all drugs out of
the reach of children.Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC.,Whitehouse Station, NJ 08889, USA
Manuf. by: Alkermes Pharma Ireland Limited, Athlone, Ireland
Aprepitant (active ingred.) Made in Switzerland.
Formulated in Ireland.
-
PRINCIPAL DISPLAY PANEL - 125 mg Capsule Carton
NDC 0006-0462-06
125 mg
EMEND®
(APREPITANT) CAPSULESFor Adults and
Pediatric Patients
12 years of Age and OlderEach capsule contains 125 mg aprepitant.
Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature].
USUAL DOSAGE: See accompanying circular.Rx only
This is a bulk package and not intended for dispensing.
Package not child resistant.6 capsules
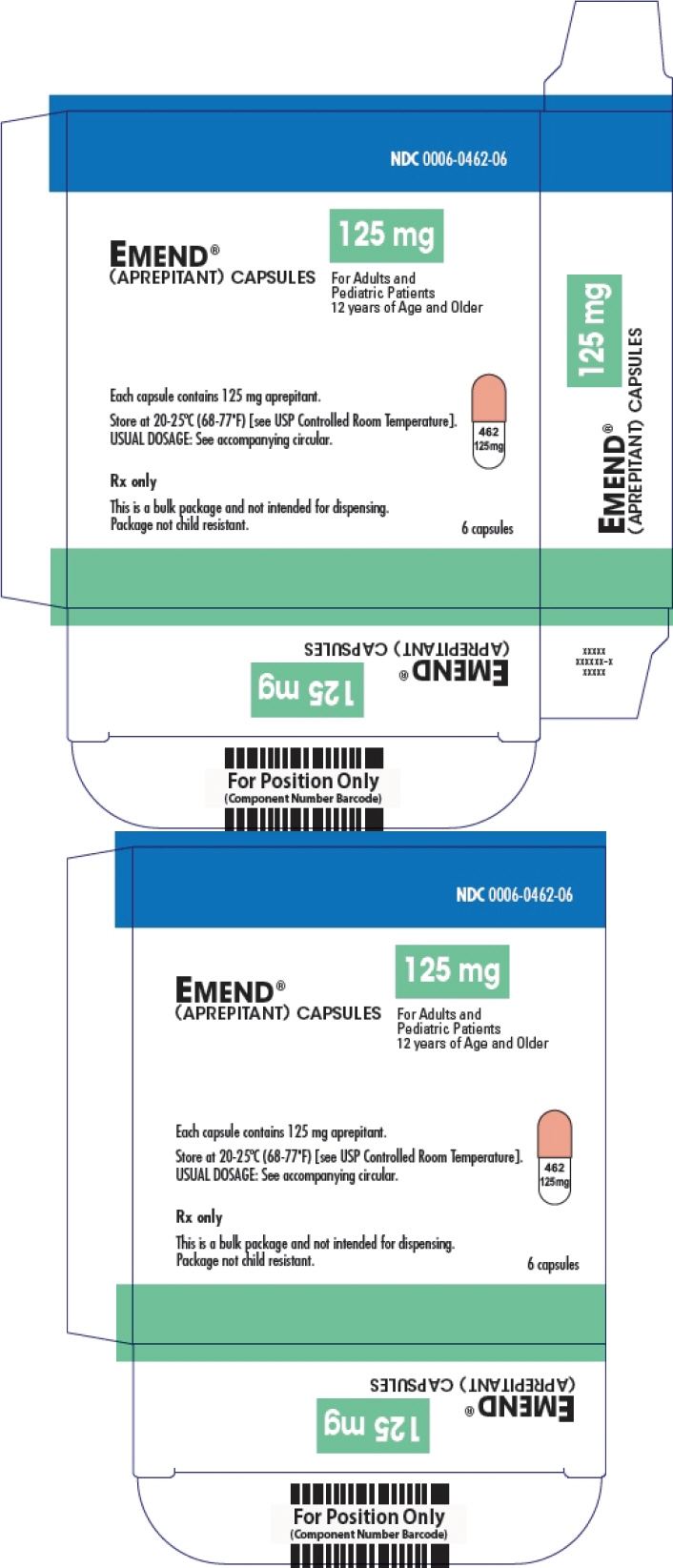
-
PRINCIPAL DISPLAY PANEL - 3 Capsule Kit Carton
NDC: 0006-3862-03
TriPack
3 capsulesEMEND®
(aprepitant) capsules125 mg 80 mg
For Adults and
Pediatric Patients
12 years of Age and OlderOne 125-mg capsule contains
125 mg aprepitant.Two 80-mg capsules each containing
80 mg of aprepitant.USUAL DOSAGE:
See accompanying circular.Rx only
Keep this and all drugs out of
the reach of children.Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Manuf. by: Alkermes Pharma Ireland Limited, Athlone, Ireland
Aprepitant (active ingred.) Made in Switzerland.
Formulated in Ireland.
-
PRINCIPAL DISPLAY PANEL - 40 mg Capsule Carton
NDC 0006-0464-10
40 mg
EMEND®
(APREPITANT) CAPSULEEach capsule contains 40 mg aprepitant.
Rx onlyPackage not child resistant. Keep this
and all drugs out of the reach of children.Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Manuf. by: Alkermes Pharma Ireland Limited, Athlone, Ireland
Formulated in Ireland1 Capsule
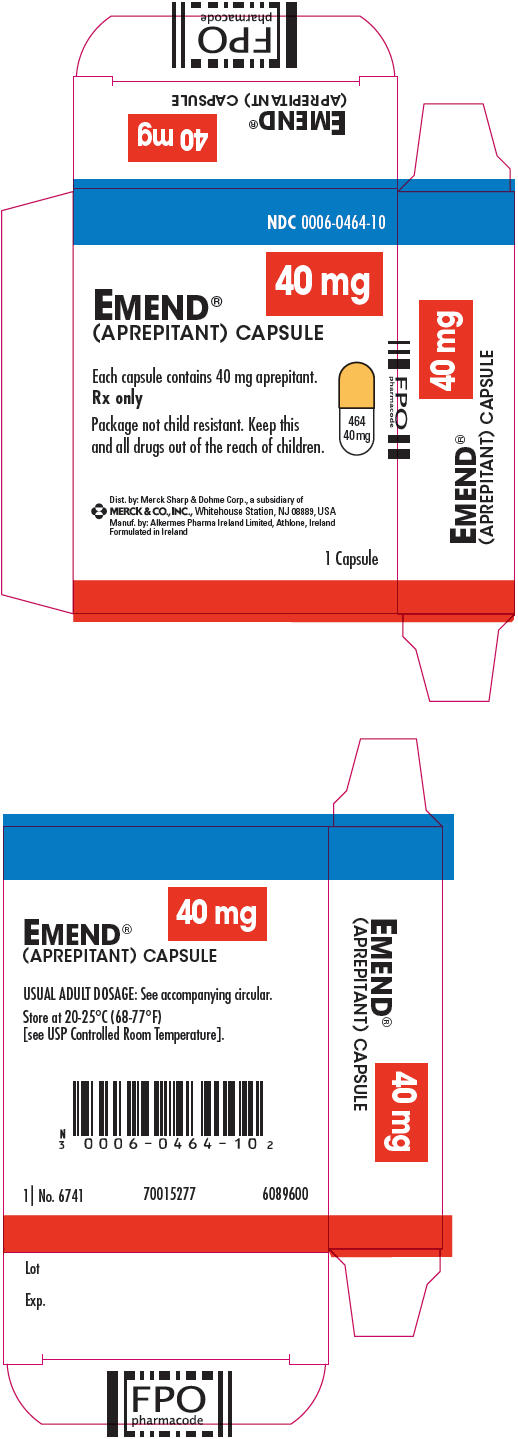
-
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC: 0006-3066-03
EMEND®
(aprepitant)
for oral
suspension125 mg
Single-Dose Kit
Discard Unused Portion
This product must be
reconstituted and dose
must be measured by a
healthcare provider.See package insert and
accompanying directions.For Oral
Administration OnlyRx only
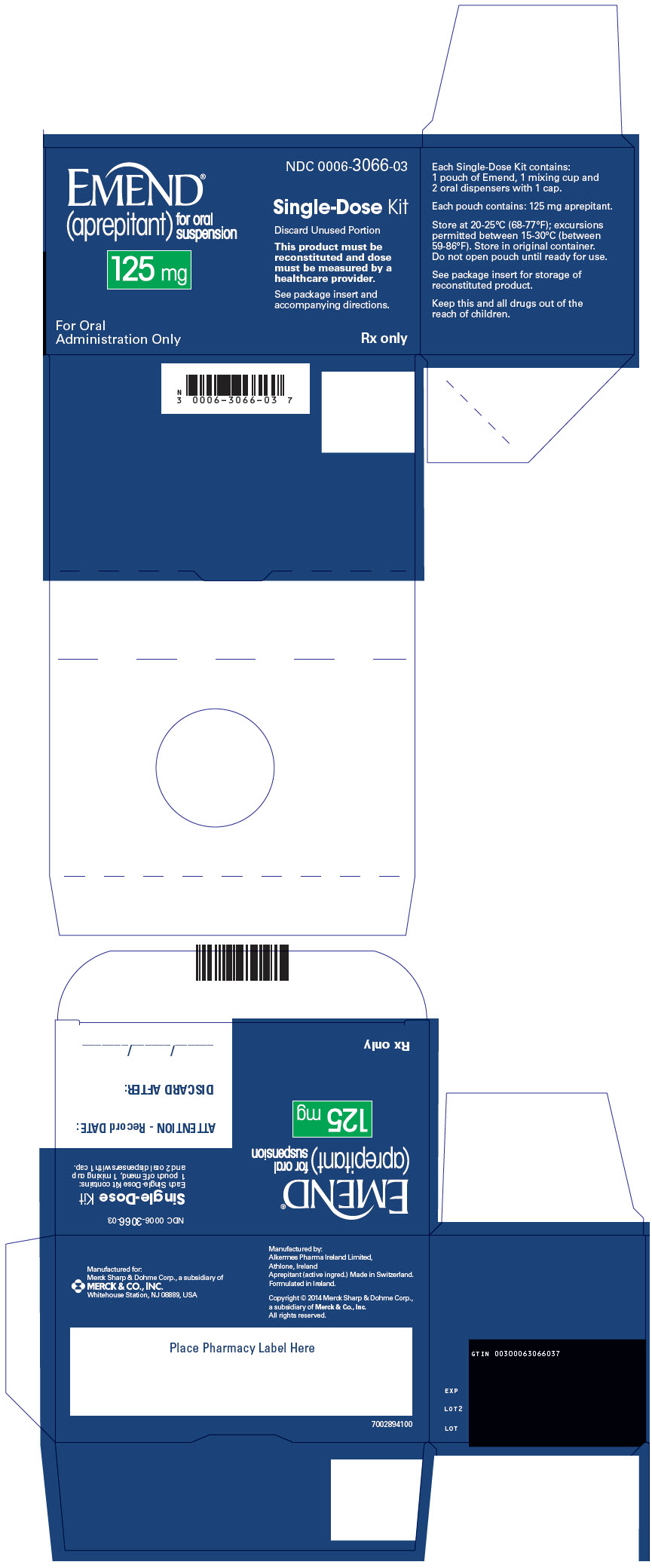
-
INGREDIENTS AND APPEARANCE
EMEND
aprepitant capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-0461 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength aprepitant (UNII: 1NF15YR6UY) (aprepitant - UNII:1NF15YR6UY) aprepitant 80 mg Inactive Ingredients Ingredient Name Strength sucrose (UNII: C151H8M554) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) sodium lauryl sulfate (UNII: 368GB5141J) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) titanium dioxide (UNII: 15FIX9V2JP) silicon dioxide (UNII: ETJ7Z6XBU4) Product Characteristics Color WHITE (white, opaque) Score no score Shape CAPSULE (capsule) Size 17mm Flavor Imprint Code 461;80;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-0461-02 2 in 1 DOSE PACK; Type 0: Not a Combination Product 03/26/2003 2 NDC: 0006-0461-06 1 in 1 CARTON 03/26/2003 04/30/2020 2 NDC: 0006-0461-01 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 0006-0461-12 2 in 1 DOSE PACK; Type 0: Not a Combination Product 03/26/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021549 03/26/2003 EMEND
aprepitant capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-0462 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength aprepitant (UNII: 1NF15YR6UY) (aprepitant - UNII:1NF15YR6UY) aprepitant 125 mg Inactive Ingredients Ingredient Name Strength sucrose (UNII: C151H8M554) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) sodium lauryl sulfate (UNII: 368GB5141J) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) titanium dioxide (UNII: 15FIX9V2JP) silicon dioxide (UNII: ETJ7Z6XBU4) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) Product Characteristics Color WHITE (opaque, white) , PINK (opaque, pink) Score no score Shape CAPSULE (capsule) Size 18mm Flavor Imprint Code 462;125;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-0462-06 1 in 1 CARTON 03/26/2003 05/31/2020 1 NDC: 0006-0462-01 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021549 03/26/2003 EMEND
aprepitant kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-3862 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-3862-03 1 in 1 DOSE PACK 03/26/2003 2 NDC: 0006-3862-13 1 in 1 DOSE PACK 03/26/2003 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 CAPSULE 2 Part 2 1 CAPSULE 1 Part 1 of 2 EMEND
aprepitant capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength aprepitant (UNII: 1NF15YR6UY) (aprepitant - UNII:1NF15YR6UY) aprepitant 80 mg Inactive Ingredients Ingredient Name Strength sucrose (UNII: C151H8M554) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) sodium lauryl sulfate (UNII: 368GB5141J) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) titanium dioxide (UNII: 15FIX9V2JP) silicon dioxide (UNII: ETJ7Z6XBU4) Product Characteristics Color WHITE (white, opaque) Score no score Shape CAPSULE (capsule) Size 17mm Flavor Imprint Code 461;80;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CAPSULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021549 03/26/2003 Part 2 of 2 EMEND
aprepitant capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength aprepitant (UNII: 1NF15YR6UY) (aprepitant - UNII:1NF15YR6UY) aprepitant 125 mg Inactive Ingredients Ingredient Name Strength sucrose (UNII: C151H8M554) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) sodium lauryl sulfate (UNII: 368GB5141J) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) titanium dioxide (UNII: 15FIX9V2JP) silicon dioxide (UNII: ETJ7Z6XBU4) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) Product Characteristics Color WHITE (opaque, white) , PINK (opaque, pink) Score no score Shape CAPSULE (capsule) Size 18mm Flavor Imprint Code 462;125;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CAPSULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021549 03/26/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021549 03/26/2003 EMEND
aprepitant capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-0464 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength aprepitant (UNII: 1NF15YR6UY) (aprepitant - UNII:1NF15YR6UY) aprepitant 40 mg Inactive Ingredients Ingredient Name Strength sucrose (UNII: C151H8M554) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) sodium lauryl sulfate (UNII: 368GB5141J) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) titanium dioxide (UNII: 15FIX9V2JP) silicon dioxide (UNII: ETJ7Z6XBU4) ferric oxide yellow (UNII: EX438O2MRT) Product Characteristics Color WHITE (opaque, white) , YELLOW (opaque, mustard yellow) Score no score Shape CAPSULE (capsule) Size 14mm Flavor Imprint Code 464;40;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-0464-10 1 in 1 CARTON 03/26/2003 02/28/2019 1 NDC: 0006-0464-01 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 0006-0464-05 5 in 1 CARTON 03/26/2003 10/31/2020 2 NDC: 0006-0464-01 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021549 03/26/2003 EMEND
aprepitant powder, for suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-3066 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength aprepitant (UNII: 1NF15YR6UY) (aprepitant - UNII:1NF15YR6UY) aprepitant 125 mg Inactive Ingredients Ingredient Name Strength sucrose (UNII: C151H8M554) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) sodium lauryl sulfate (UNII: 368GB5141J) FERRIC OXIDE RED (UNII: 1K09F3G675) sodium stearyl fumarate (UNII: 7CV7WJK4UI) Product Characteristics Color PINK (pink to light pink) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-3066-03 1 in 1 CARTON 12/17/2015 1 NDC: 0006-3066-01 1 in 1 POUCH; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207865 12/17/2015 Labeler - Merck Sharp & Dohme Corp. (001317601)
Trademark Results [EMEND]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EMEND 98452421 not registered Live/Pending |
EMEND, LLC 2024-03-15 |
 EMEND 90879357 not registered Live/Pending |
EMEND, LLC 2021-08-12 |
 EMEND 90879262 not registered Live/Pending |
EMEND, LLC 2021-08-12 |
 EMEND 90816895 not registered Live/Pending |
EMEND, LLC 2021-07-08 |
 EMEND 88511573 not registered Live/Pending |
Lonza Ltd. 2019-07-12 |
 EMEND 78449789 3154890 Live/Registered |
Medion Corporation 2004-07-13 |
 EMEND 76254977 2725335 Dead/Cancelled |
Merck & Co., Inc. 2001-05-10 |
 EMEND 75292656 2417822 Live/Registered |
MERCK & CO., Inc. 1997-05-15 |
 EMEND 74619649 2032756 Dead/Cancelled |
CLINITEX MEDICAL CORPORATION 1995-01-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.