albuterol- Albuterol aerosol, metered
Drug Labeling and Warnings
Drug Details [pdf]
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
The active component of Albuterol Inhalation Aerosol is albuterol (α1-[(tert-butylamino)methyl]-4-hydroxy-m-xylene-α,α'-diol), a relatively selective beta2-adrenergic bronchodilator, having the following structural formula:
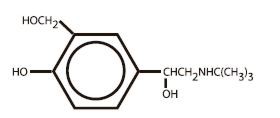
Albuterol is the official generic name in the United States. The World Health Organization recommended name for the drug is salbutamol. The molecular weight of albuterol is 239.2, and the molecular formula is C13H21NO3. Albuterol is a white to off-white crystalline solid. It is soluble in ethanol, sparingly soluble in water, and very soluble in chloroform.
Albuterol Inhalation Aerosol is a pressurized metered-dose aerosol unit for oral inhalation. It contains a microcrystalline (95% ≤ 10 µm) suspension of albuterol in propellants (trichloromonofluoromethane and dichlorodifluoromethane) with oleic acid. Each actuation delivers 100 mcg of albuterol from the valve and 90 mcg of albuterol from the mouthpiece. Each canister provides 200 inhalations.
-
CLINICAL PHARMACOLOGY
In vitro studies and in vivo pharmacologic studies have demonstrated that albuterol has a preferential effect on beta2-adrenergic receptors compared with isoproterenol. While it is recognized that beta2-adrenergic receptors are the predominant receptors in bronchial smooth muscle, data indicate that there is a population of beta2-receptors in the human heart existing in a concentration between 10% and 50%. The precise function of these receptors has not been established.
The pharmacologic effects of beta-adrenergic agonist drugs, including albuterol, are at least in part attributable to stimulation through beta-adrenergic receptors of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3', 5'- adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels are associated with relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
Albuterol has been shown in most controlled clinical trials to have more effect on the respiratory tract, in the form of bronchial smooth muscle relaxation, than isoproterenol at comparable doses while producing fewer cardiovascular effects. Controlled clinical studies and other clinical experience have shown that inhaled albuterol, like other beta-adrenergic agonist drugs, can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes.
Albuterol is longer acting than isoproterenol in most patients by any route of administration because it is not a substrate for the cellular uptake processes for catecholamines nor for catechol-O-methyl transferase.
The effects of rising doses of albuterol and isoproterenol aerosols were studied in volunteers and asthmatic patients. Results in normal volunteers indicated that albuterol is one half to one quarter as active as isoproterenol in producing increases in heart rate. In asthmatic patients similar cardiovascular differentiation between the two drugs was also seen.
Preclinical
Intravenous studies in rats with albuterol sulfate have demonstrated that albuterol crosses the blood brain barrier and reaches brain concentrations amounting to approximately 5.0% of the plasma concentrations. In structures outside the brain barrier (pineal and pituitary glands), albuterol concentrations were found to be 100 times those in the whole brain.
Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of cardiac arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta-agonists and methylxanthines are administered concurrently. The clinical significance of these findings is unknown.
Pharmacokinetics
Because of its gradual absorption from the bronchi, systemic levels of albuterol are low after inhalation of recommended doses. Studies undertaken with four subjects administered tritiated albuterol resulted in maximum plasma concentrations occurring within two to four hours. Due to the sensitivity of the assay method, the metabolic rate and half-life of elimination of albuterol in plasma could not be determined. However, urinary excretion provided data indicating that albuterol has an elimination half-life of 3.8 hours. Approximately 72% of the inhaled dose is excreted within 24 hours in the urine, and consists of 28% as unchanged drug and 44% as metabolite.
Clinical Trials
In controlled clinical trials involving adults with asthma, the onset of improvement in pulmonary function was within 15 minutes, as determined by both MMEF (maximum midexpiratory flow rate) and FEV1 (forced expiratory volume in one second). MMEF measurements also showed that near maximum improvement in pulmonary function generally occurs within 60 to 90 minutes following two inhalations of albuterol and that clinically significant improvement generally continues for three to four hours in most patients. Some patients showed a therapeutic response (defined by maintaining FEV1 values 15% or more above baseline) that was still apparent at 6 hours. Continued effectiveness of albuterol was demonstrated over a 13-week period in these same trials.
In controlled clinical trials involving children 4 to 12 years of age, FEV1 measurements showed that maximum improvement in pulmonary function occurs within 30 to 60 minutes. The onset of clinically significant (≥15%) improvement in FEV1 was observed as soon as five minutes following 180 mcg of albuterol in 18 of 30 (60%) children in a controlled dose-ranging study. Clinically significant improvement in FEV1 continued in the majority of patients for two hours and in 33% to 47% for four hours among 56 patients receiving inhalation aerosol in one pediatric study. In a second study among 48 patients receiving inhalation aerosol, clinically significant improvement continued in the majority for up to one hour and in 23% to 40% for four hours. In addition, at least 50% of the patients in both studies achieved an improvement in FEF25%–75% (forced expiratory flow rate between 25% and 75% of the forced vital capacity) of at least 20% for 2 to 5 hours. Continued effectiveness of albuterol was demonstrated over the 12-week study period.
In other clinical studies in adults and children, two inhalations of albuterol aerosol taken approximately 15 minutes before exercise prevented exercise-induced bronchospasm, as demonstrated by the maintenance of FEV1 within 80% of baseline values in the majority of patients. One study in adults also evaluated the duration of the prophylactic effect to repeated exercise challenges, which was evident at 4 hours in a majority of the patients and at 6 hours in approximately one third of the patients.
-
INDICATIONS AND USAGE
Albuterol Inhalation Aerosol is indicated for the prevention and relief of bronchospasm in patients 4 years of age and older with reversible obstructive airway disease, and for the prevention of exercise induced bronchospasm in patients 4 years of age and older.
Albuterol Inhalation Aerosol can be used with or without concomitant steroid therapy.
- CONTRAINDICATIONS
-
WARNINGS
Paradoxical Bronchospasm
Albuterol can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs, Albuterol Inhalation Aerosol should be discontinued immediately and alternative therapy instituted. It should be recognized that paradoxical bronchospasm, when associated with inhaled formulations, frequently occurs with the first use of a new canister or vial.
Cardiovascular Effects
Albuterol, like all other beta-adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of Albuterol Inhalation Aerosol at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, albuterol, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Deterioration of Asthma
Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of Albuterol Inhalation Aerosol than usual, this may be a marker of destabilization of asthma and requires reevaluation of the patient and treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
Use of Anti-Inflammatory Agents
The use of beta-adrenergic agonist bronchodilators alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents, e.g., corticosteroids.
Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions may occur after administration of Albuterol Inhalation Aerosol, as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema.
The contents of Albuterol Inhalation Aerosol are under pressure. Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw container into fire or incinerator. Avoid spraying in eyes. Keep out of reach of children.
-
PRECAUTIONS
General
Albuterol, as with all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension; in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus; and in patients who are unusually responsive to sympathomimetic amines. Clinically significant changes in systolic and diastolic blood pressure have been seen in individual patients and could be expected to occur in some patients after use of any beta-adrenergic bronchodilator.
Large doses of intravenous albuterol have been reported to aggravate preexisting diabetes mellitus and ketoacidosis. As with other beta-agonists, albuterol may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease is usually transient, not requiring supplementation.
Although there have been no reports concerning the use of Albuterol Inhalation Aerosol during labor and delivery, it has been reported that high doses of albuterol administered intravenously inhibit uterine contractions. Although this effect is extremely unlikely as a consequence of aerosol use, it should be kept in mind.
Information for Patients
The action of Albuterol Inhalation Aerosol may last up to six hours or longer. Albuterol Inhalation Aerosol should not be used more frequently than recommended. Do not increase the dose or frequency of Albuterol Inhalation Aerosol without consulting your physician. If you find that treatment with Albuterol Inhalation Aerosol becomes less effective for symptomatic relief, your symptoms become worse, and/or you need to use the product more frequently than usual, you should seek medical attention immediately. While you are using Albuterol Inhalation Aerosol, other inhaled drugs and asthma medications should be taken only as directed by your physician. Common adverse effects include palpitations, chest pain, rapid heart rate, and tremor or nervousness. If you are pregnant or nursing, contact your physician about use of Albuterol Inhalation Aerosol. Effective and safe use of Albuterol Inhalation Aerosol includes an understanding of the way that it should be administered.
In general, the technique for administering Albuterol Inhalation Aerosol to children is similar to that for adults, since children's smaller ventilatory exchange capacity automatically provides proportionally smaller aerosol intake. Children should use Albuterol Inhalation Aerosol under adult supervision, as instructed by the patient's physician.
See illustrated Patient's Instructions for Use.
Drug Interactions
Other short-acting sympathomimetic aerosol bronchodilators should not be used concomitantly with albuterol. If additional adrenergic drugs are to be administered by any route, they should be used with caution to avoid deleterious cardiovascular effects.
Monoamine Oxidase Inhibitors or Tricyclic Antidepressants
Albuterol should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within two weeks of discontinuation of such agents, because the action of albuterol on the vascular system may be potentiated.
Beta-Blockers
Beta-adrenergic receptor blocking agents not only block the pulmonary effect of beta-agonists, such as albuterol, but may produce severe bronchospasm in asthmatic patients. Therefore, patients with asthma should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents in patients with asthma. In this setting, cardioselective beta-blockers should be considered, although they should be administered with caution.
Diuretics
The ECG changes and/or hypokalemia that may result from the administration of nonpotassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of beta-agonists with nonpotassium-sparing diuretics.
Digoxin
Mean decreases of 16% to 22% in serum digoxin levels were demonstrated after single-dose intravenous and oral administration of albuterol, respectively, to normal volunteers who had received digoxin for 10 days. The clinical significance of these findings for patients with obstructive airway disease who are receiving albuterol and digoxin on a chronic basis is unclear. Nevertheless, it would be prudent to carefully evaluate the serum digoxin levels in patients who are currently receiving digoxin and albuterol.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a two-year study in Sprague-Dawley rats, albuterol sulfate caused a significant dose-related increase in the incidence of benign leiomyomas of the mesovarium at dietary doses of 2.0, 10, and 50 mg/kg (approximately 15, 70, and 340 times, respectively, the maximum recommended daily inhalation dose for adults on a mg/m2 basis, or, approximately 6, 30, and 160 times, respectively, the maximum recommended daily inhalation dose in children on a mg/m2 basis). In another study this effect was blocked by the coadministration of propranolol, a non-selective beta-adrenergic antagonist. In an 18-month study in CD-1 mice, albuterol sulfate showed no evidence of tumorigenicity at dietary doses of up to 500 mg/kg (approximately 1700 times the maximum recommended daily inhalation dose for adults on a mg/m2 basis, or, approximately 800 times the maximum recommended daily inhalation dose for children on a mg/m2 basis). In a 22-month study in the Golden hamster, albuterol sulfate showed no evidence of tumorigenicity at dietary doses of up to 50 mg/kg (approximately 225 times the maximum recommended daily inhalation dose for adults on a mg/m2 basis, or, approximately 110 times the maximum recommended daily inhalation dose for children on a mg/m2 basis).
Albuterol sulfate was not mutagenic in the Ames test with or without metabolic activation using tester strains S. typhimurium TA1537, TA1538, and TA98 or E. Coli WP2, WP2uvrA, and WP67. No forward mutation was seen in yeast strain S. cerevisiae S9 nor any mitotic gene conversion in yeast strain S. cerevisiae JD1 with or without metabolic activation. Fluctuation assays in S. typhimurium TA98 and E.Coli WP2, both with metabolic activation, were negative. Albuterol sulfate was not clastogenic in a human peripheral lymphocyte assay or in an AH1 strain mouse micronucleus assay at intraperitoneal doses of up to 200 mg/kg.
Reproduction studies in rats demonstrated no evidence of impaired fertility at oral doses of up to 50 mg/kg (approximately 340 times the maximum recommended daily inhalation dose for adults on a mg/m2 basis).
Pregnancy
Teratogenic Effects
Pregnancy Category C
Albuterol sulfate has been shown to be teratogenic in mice. A study in CD-1 mice at subcutaneous (sc) doses of 0.025, 0.25, and 2.5 mg/kg (approximately 2/25, 1.0, and 8.0 times, respectively, the maximum recommended daily inhalation dose for adults on a mg/m2 basis), showed cleft palate formation in 5 of 111 (4.5%) fetuses at 0.25 mg/kg and in 10 of 108 (9.3%) fetuses at 2.5 mg/kg. The drug did not induce cleft palate formation at the lowest dose, 0.025 mg/kg. Cleft palate also occurred in 22 of 72 (30.5%) fetuses from females treated with 2.5 mg/kg of isoproterenol (positive control) sc (approximately 8 times the maximum recommended daily inhalation dose for adults on a mg/m2 basis).
A reproduction study in Stride Dutch rabbits revealed cranioschisis in 7 of 19 (37%) fetuses when albuterol sulfate was administered orally at a 50 mg/kg dose (approximately 680 times the maximum recommended daily inhalation dose for adults on a mg/m2 basis). There are no adequate and well-controlled studies in pregnant women. Albuterol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
During worldwide marketing experience, various congenital anomalies, including cleft palate and limb defects, have been rarely reported in the offspring of patients being treated with albuterol. Some of the mothers were taking multiple medications during their pregnancies. No consistent pattern of defects can be discerned, and a relationship between albuterol use and congenital anomalies has not been established.
Use in Labor and Delivery
Because of the potential for beta-agonist interference with uterine contractility, use of albuterol for relief of bronchospasm during labor should be restricted to those patients in whom the benefits clearly outweigh the risk.
Tocolysis
Albuterol has not been approved for the management of preterm labor. The benefit:risk ratio when albuterol is administered for tocolysis has not been established. Serious adverse reactions, including maternal pulmonary edema, have been reported during or following treatment of premature labor with beta2-agonists, including albuterol.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because of the potential for tumorigenicity shown for albuterol in some animal studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
The adverse reactions to albuterol are similar in nature to reactions to other sympathomimetic agents, although the incidence of certain cardiovascular effects is lower with albuterol.
Percent Incidence of Adverse Reactions in Patients ≥ 12 Years of Age in a 13-Week Clinical Trial* Percent Incidence Reaction Albuterol Isoproterenol - * A 13-week double-blind study compared albuterol and isoproterenol inhalation aerosols in 147 asthmatic patients.
Tremor <15% <15% Nausea <15% <15% Tachycardia 10% 10% Palpitations <10% <15% Nervousness <10% <15% Increased Blood Pressure <5% <5% Dizziness <5% <5% Heartburn <5% <5% Percent Incidence of Adverse Reactions in Children 4 to 11 Years of Age in a 12-Week Trial* Reaction Percent Incidence - * A 12-week double-blind trial in 104 patients aged 4 to 11 years.
Central Nervous System Headache 3% Nervousness 1% Lightheadedness <1% Tremor <1% Agitation 1% Nightmares 1% Hyperactivity 1% Aggressive Behavior 1% Gastrointestinal Nausea and/or Vomiting 6% Stomachache 3% Diarrhea 1% Oropharyngeal Throat Irritation 6% Discoloration of Teeth 1% Respiratory Epistaxis 3% Cough 2% Musculoskeletal Muscle Cramp 1% Cases of urticaria, angioedema, rash, bronchospasm, hoarseness, oropharyngeal edema, and arrhythmias (including atrial fibrillation, supraventricular tachycardia, extrasystoles) have been reported after the use of albuterol inhalation aerosol.
In addition, albuterol, like other sympathomimetic agents, can cause adverse reactions such as hypertension, angina, vertigo, central nervous system stimulation, sleeplessness, and unusual taste.
-
OVERDOSAGE
The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the symptoms listed under ADVERSE REACTIONS, e.g., seizures, angina, hypertension, hypotension, tachycardia with rates up to 200 beats/minute, arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, and sleeplessness. Hypokalemia may also occur.
As with all sympathomimetic aerosol medications, cardiac arrest and even death may be associated with abuse of Albuterol Inhalation Aerosol. Treatment consists of discontinuation of Albuterol Inhalation Aerosol together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of Albuterol Inhalation Aerosol.
The oral median lethal dose of albuterol sulfate in mice is greater than 2000 mg/kg (approximately 6800 times the maximum recommended daily inhalation dose for adults on a mg/m2 basis, or, approximately 3200 times the maximum recommended daily inhalation dose for children on a mg/m2 basis). In mature rats, the sc median lethal dose of albuterol sulfate is approximately 450 mg/kg (approximately 3000 times the maximum recommended daily inhalation dose for adults on a mg/m2 basis, or, approximately 1400 times the maximum recommended daily inhalation dose for children on a mg/m2 basis). In small young rats, the sc median lethal dose of albuterol sulfate is approximately 2000 mg/kg (approximately 14,000 times the maximum recommended daily inhalation dose for adults on a mg/m2 basis, or, approximately 6400 times the maximum recommended daily inhalation dose for children on a mg/m2 basis). The inhalation median lethal dose has not been determined in animals.
-
DOSAGE AND ADMINISTRATION
For treatment of acute episodes of bronchospasm or prevention of asthmatic symptoms, the usual dosage for adults and pediatric patients 4 years and older is two inhalations repeated every four to six hours; in some patients, one inhalation every four hours may be sufficient. More frequent administration or a larger number of inhalations are not recommended. It is recommended to "test spray" Albuterol Inhalation Aerosol into the air before using for the first time and in cases where the aerosol has not been used for a prolonged period of time.
The use of Albuterol Inhalation Aerosol can be continued as medically indicated to control recurring bouts of bronchospasm. During this time most patients gain optimal benefit from regular use of the inhaler. Safe usage for periods extending over several years has been documented.
If a previously effective dosage regimen fails to provide the usual response, this may be a marker of destabilization of asthma and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
-
HOW SUPPLIED
Albuterol Inhalation Aerosol is supplied in 17 g canisters containing 200 metered inhalations (NDC: 17270-721-01) in boxes of one. Each actuation delivers 100 mcg of albuterol from the valve and 90 mcg of albuterol from the mouthpiece. Each canister is supplied with a blue oral adapter and patient's instructions. Also available, Albuterol Inhalation Aerosol Refill with patient's instructions (NDC: 17270-721-02).
The blue adapter supplied with Albuterol Inhalation Aerosol should not be used with any other product canisters, and the adapters from other products should not be used with an Albuterol Inhalation Aerosol canister. The correct amount of medication in each canister cannot be assured after 200 actuations, even though the canister is not completely empty. The canister should be discarded when the labeled number of actuations have been used.
Store between 15°–30°C (59°–86°F). As with most inhaled medications in aerosol canisters, the therapeutic effect of this medication may decrease when the canister is cold; for best results, the canister should be at room temperature before use.
Shake well before using.
Note: The indented statement below is required by the Federal government Clean Air Act for all products containing chlorofluorocarbons (CFC's).
- WARNING: This product contains trichloromonofluoro-methane and dichlorodifluoromethane (CFC's), substances which harm public health and environment by destroying ozone in the upper atmosphere.
A notice similar to the above warning has been placed in the patient instruction leaflet of this package insert pursuant to the regulations of the U.S. Environmental Protection Agency (EPA). The patient's warning states that the patient should consult his or her physician if there are questions about alternatives.
- SPL UNCLASSIFIED SECTION
-
Patient's Instructions for Use
Before using your Albuterol Inhalation Aerosol, read complete instructions carefully.
Children should use Albuterol Inhalation Aerosol under adult supervision, as instructed by the patient's doctor.
The blue adapter supplied with Albuterol Inhalation Aerosol should not be used with any other product canisters, and adapters from other products should not be used with an Albuterol Inhalation Aerosol canister.
The refill canister is to be used only with the blue Albuterol Inhalation Aerosol adapter ID#720.
- 1.
SHAKE THE INHALER WELL immediately before each use. Then remove the cap from the mouthpiece. Should the cap be dislodged or lost, the inhaler mouthpiece should be inspected for the presence of foreign objects before each use. Make sure the canister is fully and firmly inserted into the actuator.
- 2. BREATHE OUT FULLY THROUGH THE MOUTH, expelling as much air from your lungs as possible. Place the mouthpiece fully into the mouth, holding the inhaler in its upright position (see Figure 1) and closing the lips around it.
- 3. WHILE BREATHING IN DEEPLY AND SLOWLY THROUGH THE MOUTH, FULLY DEPRESS THE TOP OF THE METAL CANISTER with your index finger. (See Figure 2).
- 4.
HOLD YOUR BREATH AS LONG AS POSSIBLE. Before breathing out, remove the inhaler from your mouth and release your finger from the canister.
- 5. Wait one minute and SHAKE the inhaler again. Repeat steps 2 through 4 for each inhalation prescribed by your doctor.
- 6. CLEANSE THE INHALER THOROUGHLY AND FREQUENTLY. Remove the metal canister and cleanse the plastic case and cap by rinsing thoroughly in warm, running water, at least once a day. After thoroughly drying the plastic case and cap, gently replace the canister into the case with a twisting motion and put the cap back onto the mouthpiece.
- 7. As with all aerosol medications, it is recommended to "test spray" into the air before using for the first time and in cases where the aerosol has not been used for a prolonged period of time.
- 8. DISCARD THE CANISTER AFTER YOU HAVE USED THE LABELED NUMBER OF INHALATIONS.
The correct amount of medication in each inhalation cannot be assured after 200 actuations, even though the canister is not completely empty. The canister should be discarded when the labeled number of actuations have been used. Before you reach the specific number of actuations, you should consult your doctor to determine whether a refill is needed. Just as you should not take extra doses without consulting your doctor, you also should not stop using Albuterol Inhalation Aerosol without consulting your doctor. - 5. Wait one minute and SHAKE the inhaler again. Repeat steps 2 through 4 for each inhalation prescribed by your doctor.
DOSAGEUse only as directed by your doctor.
WARNINGSThe action of Albuterol Inhalation Aerosol may last up to 6 hours or longer. Albuterol Inhalation Aerosol should not be used more frequently than recommended. Do not increase the dose or frequency of Albuterol Inhalation Aerosol without consulting your doctor. If you find that treatment with Albuterol Inhalation Aerosol becomes less effective for symptomatic relief, your symptoms become worse, and/or you need to use the product more frequently than usual, you should seek medical attention immediately. While you are using Albuterol Inhalation Aerosol, other inhaled drugs and asthma medicines should be used only as directed by your doctor.
Contents Under Pressure. Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw container into fire or incinerator. Keep out of reach of children. Avoid spraying in eyes.
Store between 15° – 30°C (59°– 86°F). As with most inhaled medications in aerosol canisters, the therapeutic effect of this medication may decrease when the canister is cold; for best results, the canister should be at room temperature before use.
Shake well before using.
- 1.
SHAKE THE INHALER WELL immediately before each use. Then remove the cap from the mouthpiece. Should the cap be dislodged or lost, the inhaler mouthpiece should be inspected for the presence of foreign objects before each use. Make sure the canister is fully and firmly inserted into the actuator.
-
SPL UNCLASSIFIED SECTION
Note: The indented statement below is required by the Federal government Clean Air Act for all products containing chlorofluorocarbons (CFC's).
- This product contains trichloromonofluoromethane and dichlorodifluoromethane (CFC's), substances which harm the environment by depleting ozone in the upper atmosphere.
Your doctor has determined that this product is likely to help your personal health. USE THIS PRODUCT AS DIRECTED, UNLESS INSTRUCTED TO DO OTHERWISE BY YOUR DOCTOR. If you have any questions about alternatives, consult your doctor.
Manufactured byArmstrong Pharmaceuticals, Inc.
West Roxbury, MA 02132
An Amphastar Company
Rev. 4/06 F068B -
INGREDIENTS AND APPEARANCE
ALBUTEROL
albuterol aerosol, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17270-721 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Albuterol (UNII: QF8SVZ843E) (Albuterol - UNII:QF8SVZ843E) 90 ug Inactive Ingredients Ingredient Name Strength trichloromonoflueormethane () 60.9 mg in 1 dichlorofluoromethane (UNII: 7GAO4CRJ0B) 25.97 mg in 1 oleic acid (UNII: 2UMI9U37CP) 0.011 mg in 1 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17270-721-02 200 in 1 CANISTER Labeler - Armstrong Pharmaceuticals, Inc.
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.