ACTHIB- haemophilus influenzae type b strain 1482 capsular polysaccharide tetanus toxoid conjugate antigen kit
ActHIB by
Drug Labeling and Warnings
ActHIB by is a Other medication manufactured, distributed, or labeled by Sanofi Pasteur Inc., Sanofi Pasteur SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ActHIB® safely and effectively. See full prescribing information for ActHIB.
ActHIB [Haemophilus b Conjugate Vaccine (Tetanus Toxoid Conjugate)] Solution for Intramuscular Injection
Initial U.S. Approval: 1993INDICATIONS AND USAGE
- ActHIB is a vaccine indicated for the prevention of invasive disease caused by Haemophilus influenzae type b. ActHIB vaccine is approved for use as a four dose series in infants and children 2 months through 5 years of age (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Solution for injection: lyophilized powder to be reconstituted in supplied 0.4% Sodium Chloride diluent. A single dose, after reconstitution is 0.5 mL (3)
CONTRAINDICATIONS
- Severe allergic reaction (e.g., anaphylaxis) after a previous dose of any Haemophilus influenzae type b or tetanus toxoid-containing vaccine or any component of ActHIB vaccine. (4)
WARNINGS AND PRECAUTIONS
- If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the potential benefits and risks of giving ActHIB vaccine must be evaluated. (5.2)
ADVERSE REACTIONS
- Following administration of ActHIB vaccine in children 2-20 months of age, rates of adverse reactions varied by dose number and age of recipients:
- In children 15-20 months of age tenderness (20%) was the most common local reaction following a single dose. (6.1)
- The most frequent systemic reactions after any dose for children 2 months to 16 months of age were fussiness/irritability (75%), inconsolable crying (58%) and decreased activity/lethargy (51%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pharmacovigilance Department, Sanofi Pasteur Inc., Discovery Drive, Swiftwater, PA 18370 at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 or http://vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Immunization Series
2.2 Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
5.2 Guillain-Barré Syndrome
5.3 Altered Immunocompetence
5.4 Limitations of Vaccine Effectiveness
5.5 Tetanus Immunization
5.6 Interference with Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Other Vaccines
7.2 Immunosuppressive Treatments
7.3 Interference with Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Immunogenicity of ActHIB Vaccine in Children 2, 4, and 6 Months of Age
14.2 Immunogenicity of ActHIB Vaccine in Children 12 to 24 Months of Age
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use only
2.1 Immunization Series
ActHIB vaccine is administered as a four-dose series (0.5 mL per dose) as:
- A primary three-dose series of a single dose at 2, 4, and 6 months of age.
- A single booster dose at 15 through 18 months of age.
2.2 Reconstitution
ActHIB vaccine is a solution for injection supplied as single-dose vials of lyophilized vaccine to be reconstituted only with the accompanying saline diluent (0.4% Sodium Chloride). To reconstitute ActHIB vaccine, withdraw 0.6 mL of saline diluent and inject into the vial of lyophilized ActHIB vaccine. Agitate the vial to ensure complete reconstitution. The reconstituted ActHIB vaccine will appear clear and colorless. Withdraw a 0.5-mL dose of the reconstituted vaccine and inject intramuscularly. After reconstitution, if ActHIB vaccine is not administered promptly store at 2° to 8°C (35° to 46°F) and administer within 24 hours. Stored vaccine should be re-agitated prior to injection. Refer to Figures 1, 2, 3, and 4.
Instructions for Reconstitution of ActHIB Vaccine with Saline Diluent (0.4% Sodium Chloride) 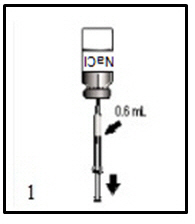
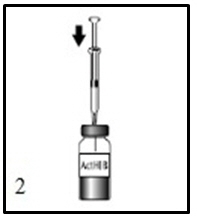

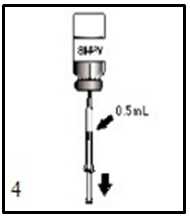
Figure 1.
Disinfect the diluent vial stopper, inject the needle and withdraw 0.6 mL of 0.4% Sodium Chloride diluent as indicated.Figure 2.
Cleanse the ActHIB vaccine stopper, insert the syringe needle into the vial, and inject the total volume of diluent.Figure 3.
Agitate vial thoroughly.Figure 4.
After reconstitution, withdraw 0.5 mL of reconstituted vaccine and administer intramuscularly.2.3 Administration
Parenteral drug products should be inspected visually for particulate matter and/or discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, the vaccine should not be administered.
ActHIB vaccine is administered as a single dose (0.5 mL) by intramuscular injection into the anterolateral aspect of the thigh or deltoid. Discard unused portion.
Do not administer this product intravenously, intradermally, or subcutaneously.
ActHIB vaccine should not be mixed in the same syringe with other parenteral products.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of any H. influenzae type b or tetanus toxoid-containing vaccine or any component of the vaccine is a contraindication to administration of ActHIB vaccine [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Epinephrine and other appropriate agents must be available should an acute anaphylactic reaction occur.
5.2 Guillain-Barré Syndrome
If Guillain-Barré syndrome has occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the decision to give any tetanus toxoid-containing vaccine, including ActHIB vaccine, should be based on careful consideration of the potential benefits and possible risks.
5.3 Altered Immunocompetence
In immunosuppressed persons, including those receiving immunosuppressive therapy, the expected antibody responses may not be obtained.
5.4 Limitations of Vaccine Effectiveness
Vaccination with ActHIB vaccine may not protect 100% of individuals.
5.5 Tetanus Immunization
Immunization with ActHIB vaccine does not substitute for routine tetanus immunization.
5.6 Interference with Laboratory Tests
Urine antigen detection may not have a diagnostic value in suspected disease due to H. influenzae type b within 1 to 2 weeks after receipt of a H. influenzae type b-containing vaccine, including ActHIB [see Drug Interactions (7.3)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
More than 7,000 infants and young children (≤2 years of age) have received at least one dose of ActHIB vaccine during US clinical trials. Of these, 1,064 subjects 12 to 24 months of age who received ActHIB vaccine alone reported no serious or life threatening adverse reactions.(1) (2)
Adverse reactions associated with ActHIB vaccine generally subsided after 24 hours and did not persist beyond 48 hours after immunization.
In a US trial, the safety of ActHIB vaccine was evaluated in 110 children 15 to 20 months of age. All children received three doses of Haemophilus influenzae type b conjugate vaccine (ActHIB vaccine or a previously licensed Haemophilus b conjugate vaccine) at approximately 2, 4, and 6 months of age. The incidence of selected solicited injection site and systemic adverse reactions which occurred within 48 hours following the dose of ActHIB vaccine is shown in Table 1.
Table 1: Local and Systemic Reactions at 6, 24, and 48 Hours Following Immunization with ActHIB Vaccine in Children 15 to 20 months old (2) Adverse Event 6 Hrs. Post-dose 24 Hrs. Post-dose 48 Hrs. Post-dose - * Induration is defined as hardness with or without swelling.
Local (%) N=110 N=110 N=110 Tenderness 20.0 8.2 0.9 Erythema
(>1")0.0 0.9 0.0 Induration* 5.5 3.6 0.9 Swelling 3.6 1.8 0.0 Systemic (%) N=103-110 N=105-110 N=104-110 Fever
(>102.2°F)
(>39.0°C)0 1.0 1.9 Irritability 27.3 20.9 12.7 Drowsiness 36.4 17.3 12.7 Anorexia 12.7 10.0 6.4 Vomiting 0.9 0.9 0.9 Persistent cry 0 0 0 Unusual cry 0 0 0 In a US clinical trial (P3T06), 1,454 children were enrolled and received one dose of ActHIB vaccine at 2 months of age and subsequent doses administered at 4 and 6 months of age (concomitantly with DAPTACEL [a US-licensed diphtheria, tetanus and pertussis vaccine], IPOL [a US-licensed inactivated poliovirus vaccine] and PCV7 [Pneumococcal conjugate vaccine, 7-valent]) vaccines at 2, 4, and 6 months of age and hepatitis B vaccine at 2 and 6 months of age). At 15-16 months of age, 418 children received a 4th dose of ActHIB and DAPTACEL vaccines. The most frequent systemic reactions following any dose (>50% of participants) were decreased activity/lethargy, fussiness/irritability, and inconsolable crying.
Table 2: Number (Percentage) of Children with Selected Solicited Systemic Adverse Reactions by Severity Occurring within 0-3 days After Vaccination in Study P3T06 Systemic Reactions DAPTACEL + IPOL + ActHIB Vaccines DAPTACEL + ActHIB Vaccines Dose 1
N=1,390-1,406
%Dose 2
N=1,346-1,360
%Dose 3
N=1,301-1,312
%Dose 4
N=379-381
%Note. - Ages of study participants ranged from 1.3 to 19.5 months. - * Fever is based upon actual temperatures recorded with no adjustments to the measurement route.
- † Following Doses 1-3 combined, the proportion of temperature measurements that were taken by axillary, rectal or other routes, or not recorded were 44.8%, 54.0%, 1.0%, and 0.1%, respectively. Following Dose 4, the proportion of temperature measurements that were taken by axillary, rectal or other routes, or not recorded were 61.1%, 36.6%, 1.7%, and 0.5%, respectively.
- ‡ Moderate: interferes with or limits usual daily activity; Severe: disabling, not interested in usual daily activity.
Fever*† ≥38.0°C 9.3 16.1 15.8 8.7 >38.5°C 1.6 4.3 5.1 3.2 >39.5°C 0.1 0.4 0.3 0.8 Decreased Activity/Lethargy‡ Any 51.1 37.4 33.2 24.1 Moderate or Severe 24.3 15.8 12.7 9.2 Severe 1.2 1.4 0.6 0.3 Inconsolable Crying Any 58.5 51.4 47.9 36.2 ≥1 hour 16.4 16.0 12.2 10.5 >3 hours 2.2 3.4 1.4 1.8 Fussiness/Irritability Any 75.8 70.7 67.1 53.8 ≥1 hour 33.3 30.5 26.2 19.4 >3 hours 5.6 5.5 4.3 4.5 In Study P3T06, within 30 days following any of Doses 1-3 of DAPTACEL + IPOL + ActHIB vaccines, 50 of 1,455 (3.4%) participants experienced a serious adverse event (SAE). One SAE of seizure with apnea occurring on the day of vaccination with the first dose of the three vaccines was determined by the investigators as possibly related. Within 30 days following Dose 4, four of 418 (1.0%) participants who received DAPTACEL + ActHIB vaccines experienced a serious adverse event. None was assessed by the investigators as related to the study of vaccines.
6.2 Postmarketing Experience
The following events have been spontaneously reported during the post-approval use of ActHIB vaccine. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
-
Immune system disorders:
Anaphylaxis, other allergic/hypersensitivity reactions (including urticaria, angioedema) -
Nervous system disorders:
Convulsions -
General disorders and administration site conditions:
Extensive limb swelling, peripheral edema, pruritus, rash (including generalized rash)
-
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Other Vaccines
In clinical trials, ActHIB vaccine was administered, at separate sites, concomitantly with one or more of the following vaccines: DTaP; Measles, Mumps and Rubella vaccine (MMR); Hepatitis B vaccine; and Inactivated Poliovirus Vaccine (IPV). No impairment of the antibody response to the individual antigens was demonstrated when ActHIB vaccine was given at the same time but separate sites with these vaccines. (2)
7.2 Immunosuppressive Treatments
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses) may reduce the immune response to ActHIB vaccine [see Altered Immunocompetence (5.3)].
7.3 Interference with Laboratory Tests
Haemophilus b capsular polysaccharide derived from Haemophilus b Conjugate Vaccines has been detected in the urine of some vaccinees. Urine antigen detection may not have a diagnostic value in suspected disease due to H. influenzae type b within 1 to 2 weeks after receipt of a H. influenzae type b-containing vaccine, including ActHIB [see Warnings and Precautions (5.6)].(3)
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
ActHIB is not approved for use in individuals 6 years of age and older. No human or animal data are available to assess vaccine-associated risks in pregnancy.
8.2 Lactation
ActHIB is not approved for use in individuals 6 years of age and older. Human or animal data are not available to assess the impact of ActHIB on milk production, its presence in breast milk, or its effects on the breastfed infant.
8.4 Pediatric Use
Safety and effectiveness of ActHIB have not been established in infants below the age of 6 weeks and children and adolescents 6 years of age and older [see Dosage and Administration (2.1)].
-
11 DESCRIPTION
ActHIB vaccine is a sterile, lyophilized powder to be reconstituted with saline diluent (0.4% Sodium Chloride) for intramuscular administration only. The vaccine consists of the Haemophilus influenzae type b capsular polysaccharide (polyribosyl-ribitol-phosphate, PRP), a high-molecular-weight polymer prepared from the H. influenzae type b strain 1482 grown in a semi-synthetic medium, covalently bound to tetanus toxoid. (4) The lyophilized ActHIB vaccine powder and saline diluent contain no preservative. The tetanus toxoid is prepared by extraction, ammonium sulfate purification, and formalin inactivation of the toxin from cultures of Clostridium tetani (Harvard strain) grown in a modified Mueller and Miller medium. (5) The culture medium contains milk-derived raw materials (casein derivatives). Further manufacturing process steps reduce residual formaldehyde to levels below 0.5 micrograms (mcg) per dose by calculation. The toxoid is filter sterilized prior to the conjugation process. Potency of ActHIB vaccine is specified on each lot by limits on the content of PRP polysaccharide and protein in each dose and the proportion of polysaccharide and protein in the vaccine that is characterized as high molecular weight conjugate.
When ActHIB is reconstituted with saline diluent (0.4% Sodium Chloride), each 0.5-mL dose is formulated to contain 10 mcg of purified capsular polysaccharide conjugated to 24 mcg of inactivated tetanus toxoid and 8.5% of sucrose.
The vial stoppers for ActHIB vaccine and diluent are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Haemophilus influenzae (H. influenzae) is a gram-negative coccobacillus. Most strains of H. influenzae that cause invasive disease (e.g., sepsis and meningitis) are H. influenzae type b.
The response to ActHIB vaccine is typical of a T-dependent immune response to antigens. The prominent isotype of anti-capsular PRP antibody induced by ActHIB vaccine is IgG. (6) A booster response for IgG has been demonstrated in children 12 months of age or older who previously received two or three doses of ActHIB vaccine. Bactericidal activity against H. influenzae type b was demonstrated in serum after immunization and correlated with the anti-PRP antibody response induced by ActHIB vaccine. (1)
Antibody titers to H. influenzae capsular polysaccharide (anti-PRP) of >1.0 mcg/mL following vaccination with unconjugated PRP vaccine correlated with long-term protection against invasive H. influenzae type b disease in children older than 24 months of age. (7) Although the relevance of this threshold to clinical protection after immunization with conjugate vaccines is not known, particularly in light of the induced, immunologic memory, this level continues to be considered as indicative of long-term protection. (8) In clinical studies, ActHIB vaccine induced, on average, anti-PRP levels ≥1.0 mcg/mL in 90% of infants after the primary series (2, 4, and 6 months) and in more than 98% of infants following a booster dose given at 15 to 19 months of age. (1)
- 13 NON-CLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Immunogenicity of ActHIB Vaccine in Children 2, 4, and 6 Months of Age
Two clinical trials supported by the National Institutes of Health (NIH) have compared the anti-PRP antibody responses to three Haemophilus influenzae type b conjugate vaccines in racially mixed populations of children. These studies were done in Tennessee (9) (Table 3) and in Minnesota, Missouri, and Texas (10) (Table 4) in infants immunized with ActHIB vaccine and other Haemophilus influenzae type b conjugate vaccines at 2, 4, and 6 months of age. All Haemophilus influenzae type b conjugate vaccines were administered concomitantly with OPV and whole-cell DTP vaccines at separate sites. Neither OPV nor whole-cell DTP vaccines are licensed or distributed in the US currently.
Table 3: Anti-PRP Antibody Responses Following a Two or Three Dose Series of a Haemophilus influenzae type b Vaccine at 2, 4, and 6 Months of Age – Tennessee (9) Vaccine N* Geometric Mean Concentration (GMC) (mcg/mL) Post Third Immunization %
≥1.0 mcg/mLPre-Immunization at 2 months Post Second Immunization at 6 months Post Third Immunization at 7 months N/A = Not applicable in this comparison trial although third dose data have been published - * N = Number of children
- † Haemophilus influenzae type b Conjugate Vaccine (Tetanus Toxoid Conjugate)
- ‡ Haemophilus influenzae type b Conjugate Vaccine (Meningococcal Protein Conjugate)
- § Seroconversion after the recommended 2-dose primary immunization series is shown
- ¶ Haemophilus influenzae type b Conjugate Vaccine (Diphtheria CRM197 Protein Conjugate)
PRP-T†
(ActHIB vaccine)65 0.10 0.30 3.64 83% PRP-OMP‡
(PedvaxHIB®)64 0.11 0.84 N/A 50%§ HbOC¶
(HibTITER®)61 0.07 0.13 3.08 75% Table 4: Anti-PRP Antibody Responses Following a Two or Three Dose Series of a Haemophilus influenzae type b Vaccine at 2, 4, and 6 Months of Age - Minnesota, Missouri, and Texas (10) Vaccine N* Geometric Mean Concentration (GMC) (mcg/mL) Post Third† Immunization %
≥1.0 mcg/mLPre-Immunization at 2 months Post Second Immunization at 6 months Post Third† Immunization at 7 months N/A = Not applicable in this comparison trial although third dose data have been published (10) - * N = Number of children
- † Sera were obtained after the third dose from 86 and 110 infants, in PRP-T and HbOC vaccine groups, respectively
- ‡ Haemophilus influenzae type b Conjugate Vaccine (Tetanus Toxoid Conjugate)
- § Haemophilus influenzae type b Conjugate Vaccine (Meningococcal Protein Conjugate)
- ¶ Seroconversion after the recommended 2-dose primary immunization series is shown
- # Haemophilus influenzae type b Conjugate Vaccine (Diphtheria CRM197 Protein Conjugate)
PRP-T‡
(ActHIB vaccine)142 0.25 1.25 6.37 97% PRP-OMP§
(PedvaxHIB)149 0.18 4.00 N/A 85%¶ HbOC#
(HibTITER)167 0.17 0.45 6.31 90% Native American populations have had high rates of H. influenzae type b disease and have been observed to have low immune responses to Haemophilus influenzae type b conjugate vaccines. In a clinical study enrolling Alaskan Native Americans, following the administration of a three-dose series of ActHIB vaccine at 6 weeks, 4 months, and 6 months of age, 75% of subjects achieved an anti-PRP antibody titer of ≥1.0 mcg/mL at 7 months of age (1 month after the last vaccination). (11)
14.2 Immunogenicity of ActHIB Vaccine in Children 12 to 24 Months of Age
In four separate studies, children 12 to 24 months of age who had not previously received Haemophilus influenzae type b conjugate vaccination were immunized with a single dose of ActHIB vaccine (Table 5). Geometric Mean Concentration (GMC) of anti-PRP antibody responses were 5.12 mcg/mL (90% responding with ≥1.0 mcg/mL) for children 12 to 15 months of age and 4.4 mcg/mL (82% responding with ≥1.0 mcg/mL) for children 17 to 24 months of age. (2)
Table 5: Anti-PRP Antibody Responses in 12- to 24-month-old Children Immunized with a Single Dose of ActHIB Age Group N* Geometric Mean Concentration (GMC) (mcg/mL) % Subjects With ≥1.0 mcg/mL Pre-Immunization Post-Immunization† Pre-Immunization Post-Immunization† - * N = Number of children
- † Post immunization responses measured at approximately 1 month after vaccination
12 to 15 months 256 0.06 5.12 1.6 90.2 17 to 24 months 81 0.10 4.40 3.7 81.5 ActHIB vaccine has been found to be immunogenic in children with sickle cell anemia, a condition that may cause increased susceptibility to Haemophilus influenzae type b disease. Following two doses of ActHIB vaccine given at two-month intervals, 89% of these children (mean age 11 months) had anti-PRP antibody titers of ≥1.0 mcg/mL. This is comparable to anti-PRP antibody levels demonstrated in children without sickle-cell anemia of similar age following two doses of ActHIB vaccine. (12)
-
15 REFERENCES
- 1 Data on file, Sanofi Pasteur SA.
- 2 Data on file, Sanofi Pasteur Inc.
- 3 Rothstein EP, et al. Comparison of antigenuria after immunization with three Haemophilus influenzae type b conjugate vaccines. Pediatr Infect Dis J 10:311-314, 1991.
- 4 Chu CY, et al. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugate. Infect Immun 40:245-246, 1983.
- 5 Mueller JH, et al. Production of diphtheria toxin of high potency (100 Lf) on a reproducible medium. J Immunol 40:21-32, 1941.
- 6 Holmes SJ, et al. Immunogenicity of four Haemophilus influenzae type b conjugate vaccines in 17- to 19-month-old children. J Pediatr 118:364-371, 1991.
- 7 Peltola H, et al. Prevention of Haemophilus influenzae type b bacteremic infections with the capsular polysaccharide vaccine. N Engl J Med 310:1561-1566, 1984.
- 8 Recommendations of the Immunization Practices Advisory Committee (ACIP). Haemophilus b conjugate vaccines for prevention of Haemophilus influenzae type b disease among infants and children two months of age and older. MMWR 40:No. RR-1, 1991.
- 9 Decker MD, et al. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr 120:184-189, 1992.
- 10 Granoff DM, et al. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J Pediatr 121:187-194, 1992.
- 11 Bulkow LR, et al. Comparative immunogenicity of four Haemophilus influenzae type b conjugate vaccines in Alaska Native infants. Pediatr Infect Dis J 12:484-92, 1993.
- 12 Kaplan SL, et al. Immunogenicity of Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine in children with sickle hemoglobinopathy or malignancies, and after systemic Haemophilus influenzae type b infection. J Pediatr 120:367-370, 1992.
- 13 Vaccine Adverse Event Reporting System United States. MMWR 39:730-733, 1990.
- 14 CDC. National Childhood Vaccine Injury Act: Requirements for permanent vaccination records and for reporting of selected events after vaccination. MMWR 37:197-200, 1988.
- 15 National Childhood Vaccine Injury Act of 1986 (Amended 1987).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Single-dose, lyophilized vaccine vial (NDC: 49281-547-58) packaged with single-dose diluent vial (NDC: 49281-546-58). Supplied as package of 5 vials each (NDC: 49281-545-03).
The vial stoppers for ActHIB vaccine and diluent are not made with natural rubber latex.
-
17 PATIENT COUNSELING INFORMATION
Vaccine Information Statements are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to immunization to the patient, parent, or guardian.
Inform the patients, parents, or guardians about the potential benefits and risks of the vaccine and importance of completing the immunization series unless a contraindication to further immunization exists. In addition to this, parents and guardians must be informed about the potential for adverse reactions that have been temporarily associated with the administration of ActHIB vaccine or other vaccines containing similar ingredients. Prior to administration of ActHIB vaccine, healthcare providers should ask parents or guardians about the recent health status of the infant or child to be immunized. As part of the child's immunization record, the date, lot number, and manufacturer of the vaccine administered should be recorded. (13) (14) (15) Vaccine recipients and guardians must report any adverse reactions upon administration of the vaccine to their healthcare provider and/or to the Vaccine Adverse Event Reporting System (VAERS).
ActHIB, DAPTACEL and IPOL are registered trademarks of Sanofi Pasteur Inc.
PedvaxHIB® is a registered trademark of Merck & Co., Inc.
HibTITER® is a registered trademark of Nuron Biotech.Product information
as of June 2019. - SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC: 49281-545-03
Hib
Haemophilus b
Conjugate Vaccine
(Tetanus Toxoid Conjugate)5 VIALS
1 Dose
eachActHIB®
Rx only
For children 2 months through 5 years of age
SANOFI PASTEUR
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Label
NDC: 49281-547-58
Hib
Haemophilus b
Conjugate Vaccine
(Tetanus Toxoid Conjugate)
1 Dose
(0.5 mL)ActHIB®
Rx only
IM onlyMfg by: Sanofi Pasteur SA
6346

-
INGREDIENTS AND APPEARANCE
ACTHIB
haemophilus influenzae type b strain 1482 capsular polysaccharide tetanus toxoid conjugate antigen kitProduct Information Product Type VACCINE Item Code (Source) NDC: 49281-545 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-545-03 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 5 VIAL, SINGLE-DOSE 2.5 mL Part 2 5 VIAL, SINGLE-DOSE 3 mL Part 1 of 2 ACTHIB
haemophilus influenzae type b strain 1482 capsular polysaccharide tetanus toxoid conjugate antigen injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 49281-547 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HAEMOPHILUS INFLUENZAE TYPE B STRAIN 1482 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN (UNII: FLV5I5W26R) (HAEMOPHILUS INFLUENZAE TYPE B STRAIN 1482 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN - UNII:FLV5I5W26R) HAEMOPHILUS INFLUENZAE TYPE B STRAIN 1482 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN 10 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength FORMALDEHYDE (UNII: 1HG84L3525) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-547-58 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103935 03/30/1993 Part 2 of 2 SALINE DILUENT
sodium chloride injection, solutionProduct Information Item Code (Source) NDC: 49281-546 Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) 4 mg in 1 mL Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-546-58 0.6 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103935 03/30/1993 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103935 03/30/1993 Labeler - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur SA 578763542 MANUFACTURE Establishment Name Address ID/FEI Business Operations Sanofi Pasteur Inc. 086723285 MANUFACTURE
Trademark Results [ActHIB]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACTHIB 75422707 2225863 Live/Registered |
SANOFI PASTEUR 1998-01-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.