PFIZER-BIONTECH COVID-19 VACCINE
Pfizer-BioNTech Covid-19 Vaccine by
Drug Labeling and Warnings
Pfizer-BioNTech Covid-19 Vaccine by is a Other medication manufactured, distributed, or labeled by Pfizer Manufacturing Belgium NV, Pfizer Inc, Pharmacia & Upjohn Company LLC, Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PFIZER-BIONTECH COVID-19 VACCINE- bnt162b2 injection, suspension
Pfizer Manufacturing Belgium NV
----------
PFIZER-BIONTECH COVID-19 VACCINE
FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS)
EMERGENCY USE AUTHORIZATION (EUA) OF THE PFIZER-BIONTECH COVID-19 VACCINE TO PREVENT CORONAVIRUS DISEASE 2019 (COVID-19)
|
PRIMARY SERIES FOR 12 YEARS OF AGE AND OLDER
|
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) to permit the emergency use of the unapproved product, Pfizer-BioNTech COVID-19 Vaccine, for active immunization to prevent COVID-19 in individuals 6 months of age and older.
There are 2 formulations of Pfizer-BioNTech COVID-19 Vaccine authorized for use in individuals 12 years of age and older:
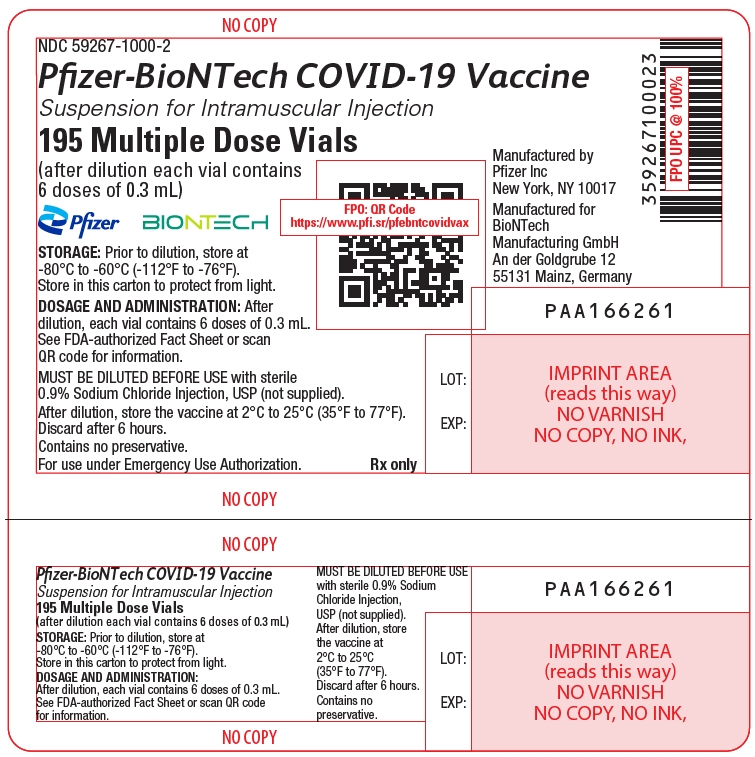
- The formulation supplied in a multiple dose vial with a purple cap MUST BE DILUTED PRIOR TO USE.
- The formulation supplied in a multiple dose vial with a gray cap and label with a gray border IS NOT DILUTED PRIOR TO USE.
This Fact Sheet pertains only to Pfizer-BioNTech COVID-19 Vaccine supplied in a multiple dose vial with a purple cap, which is authorized for use in individuals 12 years of age and older and MUST BE DILUTED PRIOR TO USE.
Pfizer-BioNTech COVID-19 Vaccine supplied in a multiple dose vial with a purple cap is authorized for use to provide:
- a 2-dose primary series to individuals 12 years of age and older; and
- a third primary series dose to individuals 12 years of age and older with certain kinds of immunocompromise.1
COMIRNATY (COVID-19 Vaccine, mRNA) is an FDA-approved COVID-19 vaccine made by Pfizer for BioNTech that is indicated for active immunization to prevent COVID-19 in individuals 12 years of age and older. It is approved for use as a 2-dose primary series for the prevention of COVID-19 in individuals 12 years of age and older. It is also authorized for emergency use to provide a third primary series dose to individuals 12 years of age and older with certain kinds of immunocompromise.
The FDA-approved COMIRNATY (COVID-19 Vaccine, mRNA) and the EUA-authorized Pfizer-BioNTech COVID-19 Vaccine for individuals 12 years of age and older when prepared according to their respective instructions for use can be used interchangeably.2
COMIRNATY (COVID-19 Vaccine, mRNA) and the Pfizer-BioNTech COVID-19 Vaccine intended for individuals 12 years of age and older should not be used for individuals 6 months through 11 years of age because of the potential for vaccine administration errors, including dosing errors.3
SUMMARY OF INSTRUCTIONS FOR COVID-19 VACCINATION PROVIDERS
Vaccination providers enrolled in the federal COVID-19 Vaccination Program must report all vaccine administration errors, all serious adverse events, cases of myocarditis, cases of pericarditis, cases of Multisystem Inflammatory Syndrome (MIS) in adults and children, and cases of COVID-19 that result in hospitalization or death following administration of Pfizer-BioNTech COVID-19 Vaccine. See "MANDATORY REQUIREMENTS FOR PFIZER-BIONTECH COVID-19 VACCINE ADMINISTRATION UNDER EMERGENCY USE AUTHORIZATION" for reporting requirements.
The Pfizer-BioNTech COVID-19 Vaccine is a suspension for intramuscular injection.
Primary Series
The Pfizer-BioNTech COVID-19 Vaccine is administered as a primary series of 2 doses (0.3 mL each) 3 weeks apart in individuals 12 years of age or older.
A third primary series dose of the Pfizer-BioNTech COVID-19 Vaccine (0.3 mL) at least 28 days following the second dose is authorized for administration to individuals at least 12 years of age with certain kinds of immunocompromise.
See this Fact Sheet for instructions for preparation and administration. This Fact Sheet may have been updated. For the most recent Fact Sheet, please see www.cvdvaccine.com.
For information on clinical trials that are testing the use of the Pfizer-BioNTech COVID-19 Vaccine for active immunization to prevent COVID-19, please see www.clinicaltrials.gov.
DESCRIPTION OF COVID-19
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the novel coronavirus, SARS-CoV-2, that appeared in late 2019. It is predominantly a respiratory illness that can affect other organs. People with COVID-19 have reported a wide range of symptoms, ranging from mild symptoms to severe illness. Symptoms may appear 2 to 14 days after exposure to the virus. Symptoms may include: fever or chills; cough; shortness of breath; fatigue; muscle or body aches; headache; new loss of taste or smell; sore throat; congestion or runny nose; nausea or vomiting; diarrhea.
DOSAGE AND ADMINISTRATION
The storage, preparation, and administration information in this Fact Sheet apply to the Pfizer-BioNTech COVID-19 Vaccine for individuals 12 years of age and older, which is supplied in a multiple dose vial with a purple cap and MUST BE DILUTED before use.
| Age Range | Dilution Information | Doses Per Vial After Dilution | Dose Volume |
|---|---|---|---|
|
12 years and older |
Dilute with 1.8 mL sterile 0.9% Sodium Chloride Injection, USP prior to use |
6 |
0.3 mL |
During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
Do not refreeze thawed vials.
Frozen Vials Prior to Use
Cartons of Pfizer-BioNTech COVID-19 Vaccine multiple dose vials with purple caps arrive in thermal containers with dry ice. Once received, remove the vial cartons immediately from the thermal container and preferably store in an ultra-low temperature freezer between -90ºC to -60ºC (-130ºF to -76ºF) until the expiry date printed on the label. This information in the package insert supersedes the storage conditions printed on the vial cartons.
Cartons and vials of Pfizer-BioNTech COVID-19 Vaccine supplied in multiple dose vials with purple caps with an expiry date of December 2021 through December 2022 printed on the label may remain in use beyond the printed date until the updated expiry date shown below; as long as approved storage conditions have been maintained.
|
Printed Expiry Date |
Updated Expiry Date |
|
|
12/2021 |
➜ |
31-Dec-2022 |
|
01/2022 |
➜ |
31-Jan-2023 |
|
02/2022 |
➜ |
28-Feb-2023 |
|
03/2022 |
➜ |
31-Mar-2023 |
|
06/2022 |
➜ |
31-Mar-2023 |
|
07/2022 |
➜ |
30-Apr-2023 |
|
08/2022 |
➜ |
31-May-2023 |
|
09/2022 |
➜ |
30-Jun-2023 |
|
10/2022 |
➜ |
31-July-2023 |
|
11/2022 |
➜ |
31-Aug-2023 |
|
12/2022 |
➜ |
30-Sep-2023 |
If not stored between -90ºC to -60ºC (-130ºF to -76ºF), vials may be stored at -25°C to -15°C (-13°F to 5°F) for up to 2 weeks. Vials must be kept frozen and protected from light until ready to use. Vials stored at -25°C to -15°C (-13°F to 5°F) for up to 2 weeks may be returned one time to the recommended storage condition of -90ºC to -60ºC (-130ºF to -76ºF). Total cumulative time the vials are stored at -25°C to -15°C (-13°F to 5°F) should be tracked and should not exceed 2 weeks.
If an ultra-low temperature freezer is not available, the thermal container in which the Pfizer-BioNTech COVID-19 Vaccine arrives may be used as temporary storage when consistently re-filled to the top of the container with dry ice. Refer to the re-icing guidelines packed in the original thermal container for instructions regarding the use of the thermal container for temporary storage. The thermal container maintains a temperature range of -90ºC to -60ºC (-130ºF to -76ºF). Storage of the vials between -96°C to -60°C (-141°F to -76°F) is not considered an excursion from the recommended storage condition.
Transportation of Frozen Vials
If local redistribution is needed and full cartons containing vials cannot be transported at -90°C to -60°C (-130°F to -76°F), vials may be transported at -25°C to -15°C (-13°F to 5°F). Any hours used for transport at -25°C to -15°C (-13°F to 5°F) count against the 2-week limit for storage at -25°C to -15°C (-13°F to 5°F). Frozen vials transported at -25°C to -15°C (-13°F to 5°F) may be returned one time to the recommended storage condition of -90ºC to -60ºC (-130ºF to -76ºF).
Thawed Vials Before Dilution
Thawed Under Refrigeration
Thaw and then store undiluted vials in the refrigerator [2ºC to 8ºC (35ºF to 46ºF)] for up to 1 month. A carton of 25 vials or 195 vials may take up to 2 or 3 hours, respectively, to thaw in the refrigerator, whereas a fewer number of vials will thaw in less time.
Thawed at Room Temperature
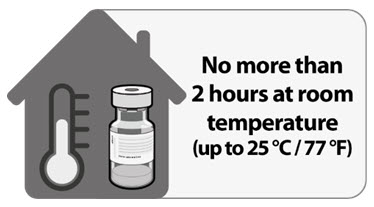
For immediate use, thaw undiluted vials at room temperature [up to 25ºC (77ºF)] for 30 minutes. Thawed vials can be handled in room light conditions. Vials must reach room temperature before dilution.
Undiluted vials may be stored at room temperature for no more than 2 hours.
Transportation of Thawed Vials
Available data support transportation of one or more thawed vials at 2°C to 8°C (35°F to 46°F) for up to 48 hours.
Vials After Dilution
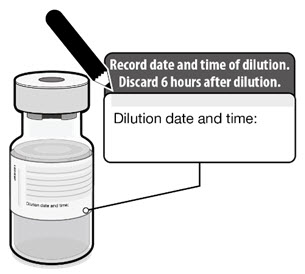
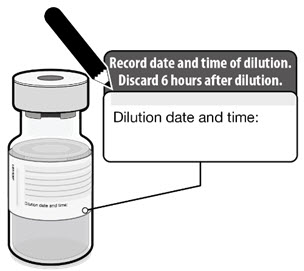
- After dilution, store vials between 2°C to 25°C (35°F to 77°F) and use within 6 hours from the time of dilution.
- During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
- Any vaccine remaining in vials must be discarded after 6 hours.
- Do not refreeze.
Dosing and Schedule
Primary Series
The Pfizer-BioNTech COVID-19 Vaccine is administered intramuscularly as a primary series of 2 doses (0.3 mL each) 3 weeks apart to individuals 12 years of age and older.
A third primary series dose of the Pfizer-BioNTech COVID-19 Vaccine (0.3 mL) at least 28 days following the second dose is authorized for administration to individuals at least 12 years of age with certain kinds of immunocompromise.
Dose Preparation
Each vial MUST BE DILUTED before administering the vaccine.
Prior to Dilution
- The Pfizer-BioNTech COVID-19 Vaccine multiple dose vial with a purple cap contains a volume of 0.45 mL and is supplied as a frozen suspension that does not contain preservative.
- Each vial must be thawed before dilution.
Dilution
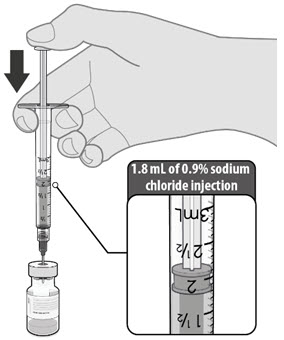
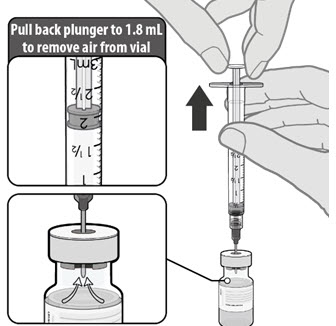
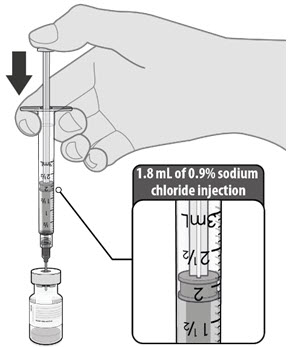
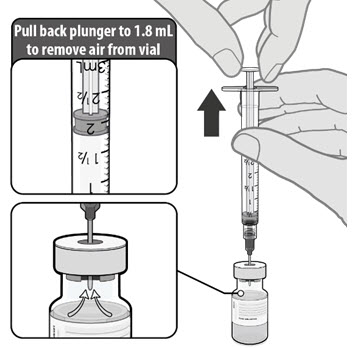
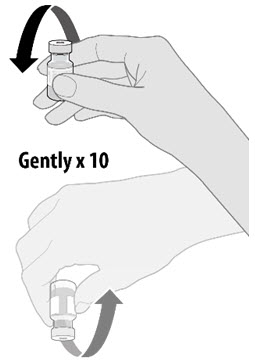
Dilute the vial contents using 1.8 mL of sterile 0.9% Sodium Chloride Injection, USP (not provided) to form the Pfizer-BioNTech COVID-19 Vaccine. ONLY use sterile 0.9% Sodium Chloride Injection, USP as the diluent. This diluent is not packaged with the vaccine and must be sourced separately. Do not use bacteriostatic 0.9% Sodium Chloride Injection or any other diluent. Do not add more than 1.8 mL of diluent.
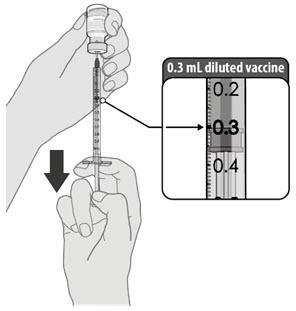
After dilution, 1 vial contains 6 doses of 0.3 mL.
Administration
Visually inspect each dose in the dosing syringe prior to administration. The vaccine will be an off-white suspension. During the visual inspection,
- verify the final dosing volume of 0.3 mL.
- confirm there are no particulates and that no discoloration is observed.
- do not administer if vaccine is discolored or contains particulate matter.
Administer the Pfizer-BioNTech COVID-19 Vaccine intramuscularly.
After dilution, vials of Pfizer-BioNTech COVID-19 Vaccine with purple caps contain 6 doses of 0.3 mL of vaccine. Low dead-volume syringes and/or needles can be used to extract 6 doses from a single vial. If standard syringes and needles are used, there may not be sufficient volume to extract 6 doses from a single vial. Irrespective of the type of syringe and needle:
- Each dose must contain 0.3 mL of vaccine.
- If the amount of vaccine remaining in the vial cannot provide a full dose of 0.3 mL, discard the vial and content.
- Do not pool excess vaccine from multiple vials.
Contraindications
Do not administer Pfizer-BioNTech COVID-19 Vaccine to individuals with known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 Vaccine (see Full EUA Prescribing Information).
Warnings
Management of Acute Allergic Reactions
Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of Pfizer-BioNTech COVID-19 Vaccine.
Monitor Pfizer-BioNTech COVID-19 Vaccine recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention (CDC) guidelines (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
Myocarditis and Pericarditis
Postmarketing data with Pfizer-BioNTech COVID-19 Vaccine demonstrate increased risks of myocarditis and pericarditis, particularly within the period 0 through 7 days following the second dose of the primary series. The observed risk is higher among adolescent males and adult males under 40 years of age than among females and older males. The observed risk is highest in males 12 through 17 years of age. Although some cases required intensive care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae. The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html).
Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines, in particular in adolescents. Procedures should be in place to avoid injury from fainting.
Altered Immunocompetence
Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to the Pfizer-BioNTech COVID-19 Vaccine.
Limitation of Effectiveness
Pfizer-BioNTech COVID-19 Vaccine may not protect all vaccine recipients.
Adverse Reactions
Adverse Reactions in Clinical Trials
Adverse reactions following administration of the Pfizer-BioNTech COVID-19 Vaccine that have been reported in clinical trials include injection site pain, fatigue, headache, muscle pain, chills, joint pain, fever, injection site swelling, injection site redness, nausea, malaise, lymphadenopathy, decreased appetite, rash, and pain in extremity (see Full EUA Prescribing Information).
Adverse Reactions Identified in Post Authorization Experience
Severe allergic reactions, including anaphylaxis, and other hypersensitivity reactions (e.g., rash, pruritus, urticaria, angioedema), diarrhea, vomiting, pain in extremity (arm), syncope, and dizziness have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine.
Myocarditis and pericarditis have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine.
Additional adverse reactions, some of which may be serious, may become apparent with more widespread use of the Pfizer-BioNTech COVID-19 Vaccine.
Use with Other Vaccines
There is no information on the co-administration of the Pfizer-BioNTech COVID-19 Vaccine with other vaccines.
INFORMATION TO PROVIDE TO VACCINE RECIPIENTS/CAREGIVERS
As the vaccination provider, you must communicate to the recipient or their caregiver, information consistent with the "Vaccine Information Fact Sheet for Recipients and Caregivers" (and provide a copy or direct the individual to the website www.cvdvaccine.com to obtain the Vaccine Information Fact Sheet for Recipients and Caregivers) prior to the individual receiving each dose of the Pfizer-BioNTech COVID-19 Vaccine, including:
- FDA has authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine, which is not an FDA-approved vaccine.
- There is an option to accept or refuse Pfizer-BioNTech COVID-19 Vaccine.
- The significant known and potential risks and benefits of the Pfizer-BioNTech COVID-19 Vaccine, and the extent to which such risks and benefits are unknown.
- Information about available alternative vaccines and the risks and benefits of those alternatives.
For information on clinical trials that are testing the use of the Pfizer-BioNTech COVID-19 Vaccine to prevent COVID-19, please see www.clinicaltrials.gov.
Provide a vaccination card to the recipient or their caregiver with the date when the recipient needs to return for the second dose of Pfizer-BioNTech COVID-19 Vaccine.
Provide the v-safe information sheet to vaccine recipients/caregivers and encourage vaccine recipients to participate in v-safe. V-safe is a new voluntary smartphone-based tool that uses text messaging and web surveys to check in with people who have been vaccinated to identify potential side effects after COVID-19 vaccination. V-safe asks questions that help CDC monitor the safety of COVID-19 vaccines. V-safe also provides dose reminders if needed and live telephone follow-up by CDC if participants report a significant health impact following COVID-19 vaccination. For more information, visit: www.cdc.gov/vsafe.
MANDATORY REQUIREMENTS FOR PFIZER-BIONTECH COVID-19 VACCINE ADMINISTRATION UNDER EMERGENCY USE AUTHORIZATION4
In order to mitigate the risks of using this unapproved product under EUA and to optimize the potential benefit of Pfizer-BioNTech COVID-19 Vaccine, the following items are required. Use of unapproved Pfizer-BioNTech COVID-19 Vaccine for active immunization to prevent COVID-19 under this EUA is limited to the following (all requirements must be met):
- 1. Pfizer-BioNTech COVID-19 Vaccine is authorized for use in individuals 6 months of age and older.
- 2. The vaccination provider must communicate to the individual receiving the Pfizer-BioNTech COVID-19 Vaccine or their caregiver, information consistent with the "Vaccine Information Fact Sheet for Recipients and Caregivers" prior to the individual receiving Pfizer-BioNTech COVID-19 Vaccine.
- 3. The vaccination provider must include vaccination information in the state/local jurisdiction's Immunization Information System (IIS) or other designated system.
- 4.
The vaccination provider is responsible for mandatory reporting of the following to the Vaccine Adverse Event Reporting System (VAERS):Complete and submit reports to VAERS online at https://vaers.hhs.gov/reportevent.html. For further assistance with reporting to VAERS call 1-800-822-7967. The reports should include the words "Pfizer-BioNTech COVID-19 Vaccine EUA" in the description section of the report.
- vaccine administration errors whether or not associated with an adverse event,
- serious adverse events* (irrespective of attribution to vaccination),
- cases of myocarditis,
- cases of pericarditis,
- cases of Multisystem Inflammatory Syndrome (MIS) in adults and children, and
- cases of COVID-19 that result in hospitalization or death.
- 5. The vaccination provider is responsible for responding to FDA requests for information about vaccine administration errors, adverse events, cases of myocarditis, cases of pericarditis, cases of MIS in adults and children, and cases of COVID-19 that result in hospitalization or death following administration of Pfizer-BioNTech COVID-19 Vaccine to recipients.
* Serious adverse events are defined as:
- Death;
- A life-threatening adverse event;
- Inpatient hospitalization or prolongation of existing hospitalization;
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions;
- A congenital anomaly/birth defect;
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent 1 of the outcomes listed above.
OTHER ADVERSE EVENT REPORTING TO VAERS AND PFIZER INC.
Vaccination providers may report to VAERS other adverse events that are not required to be reported using the contact information above.
To the extent feasible, report adverse events to Pfizer Inc. using the contact information below or by providing a copy of the VAERS form to Pfizer Inc.
| Website | Fax number | Telephone number |
|---|---|---|
|
1-866-635-8337 |
1-800-438-1985 |
ADDITIONAL INFORMATION
For general questions, visit the website or call the telephone number provided below.
To access the most recent Pfizer-BioNTech COVID-19 Vaccine Fact Sheets, please scan the QR code provided below.
| Global website | Telephone number |
|---|---|
|
1-877-829-2619 |
AVAILABLE ALTERNATIVES
COMIRNATY (COVID-19 Vaccine, mRNA) and SPIKEVAX (COVID-19 Vaccine, mRNA) are FDA-approved vaccines to prevent COVID-19 caused by SARS-CoV-2. There may be clinical trials or availability under EUA of other COVID-19 vaccines.
COMIRNATY (COVID-19 Vaccine, mRNA) and the Pfizer-BioNTech COVID-19 Vaccine intended for individuals 12 years of age and older should not be used for individuals 6 months through 11 years of age because of the potential for vaccine administration errors, including dosing errors.
FEDERAL COVID-19 VACCINATION PROGRAM
This vaccine is being made available for emergency use exclusively through the CDC COVID-19 Vaccination Program (the Vaccination Program). Healthcare providers must enroll as providers in the Vaccination Program and comply with the provider requirements. Vaccination providers may not charge any fee for the vaccine and may not charge the vaccine recipient any out-of-pocket charge for administration. However, vaccination providers may seek appropriate reimbursement from a program or plan that covers COVID-19 vaccine administration fees for the vaccine recipient (private insurance, Medicare, Medicaid, Health Resources & Services Administration [HRSA] COVID-19 Uninsured Program for non-insured recipients). For information regarding provider requirements and enrollment in the CDC COVID-19 Vaccination Program, see https://www.cdc.gov/vaccines/covid-19/provider-enrollment.html.
Individuals becoming aware of any potential violations of the CDC COVID-19 Vaccination Program requirements are encouraged to report them to the Office of the Inspector General, U.S. Department of Health and Human Services, at 1-800-HHS-TIPS or https://TIPS.HHS.GOV.
AUTHORITY FOR ISSUANCE OF THE EUA
The Secretary of Health and Human Services (HHS) has declared a public health emergency that justifies the emergency use of drugs and biological products during the COVID-19 pandemic. In response, FDA has issued an EUA for the unapproved product, Pfizer-BioNTech COVID-19 Vaccine, and for certain uses of FDA-approved COMIRNATY (COVID-19 Vaccine, mRNA) for active immunization to prevent COVID-19.
FDA issued this EUA, based on Pfizer-BioNTech's request and submitted data.
For the authorized uses, although limited scientific information is available, based on the totality of the scientific evidence available to date, it is reasonable to believe that the Pfizer-BioNTech COVID-19 Vaccine and COMIRNATY (COVID-19 Vaccine, mRNA) may be effective for the prevention of COVID-19 in individuals as specified in the Full EUA Prescribing Information.
This EUA for the Pfizer-BioNTech COVID-19 Vaccine and COMIRNATY (COVID-19 Vaccine, mRNA) will end when the Secretary of HHS determines that the circumstances justifying the EUA no longer exist or when there is a change in the approval status of the product such that an EUA is no longer needed.
For additional information about Emergency Use Authorization visit FDA at: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
The Countermeasures Injury Compensation Program
The Countermeasures Injury Compensation Program (CICP) is a federal program that has been created to help pay for related costs of medical care and other specific expenses to compensate people injured after use of certain medical countermeasures. Medical countermeasures are specific vaccines, medications, devices, or other items used to prevent, diagnose, or treat the public during a public health emergency or a security threat. For more information about CICP regarding the Pfizer-BioNTech COVID-19 Vaccine used to prevent COVID-19, visit www.hrsa.gov/cicp/, email cicp@hrsa.gov, or call: 1-855-266-2427.
Manufactured for
BioNTech Manufacturing GmbH
An der Goldgrube 12
55131 Mainz, Germany
Manufactured by
Pfizer Inc., New York, NY 10017
LAB-1450-31.0
Revised: 22 December 2022
END SHORT VERSION FACT SHEET
Long Version (Full EUA Prescribing Information) Begins On Next Page
|
FULL EMERGENCY USE AUTHORIZATION (EUA) PRESCRIBING INFORMATION | |
|
PFIZER-BIONTECH COVID-19 VACCINE | |
|
FULL EMERGENCY USE AUTHORIZATION PRESCRIBING INFORMATION: CONTENTS*
|
* Sections or subsections omitted from the full emergency use authorization prescribing information are not listed. |
1 AUTHORIZED USE
Pfizer-BioNTech COVID-19 Vaccine is authorized for use under an Emergency Use Authorization (EUA) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 6 months of age and older.
This EUA Prescribing Information pertains only to Pfizer-BioNTech COVID-19 Vaccine supplied in a multiple dose vial with a purple cap, which is authorized for use in individuals 12 years of age and older.
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
The storage, preparation, and administration information in this Prescribing Information apply to the Pfizer-BioNTech COVID-19 Vaccine for individuals 12 years of age and older, which is supplied in a multiple dose vial with a purple cap and MUST BE DILUTED before use.
| Age Range | Dilution Information | Doses Per Vial After Dilution | Dose Volume |
|---|---|---|---|
|
12 years and older |
Dilute with 1.8 mL sterile 0.9% Sodium Chloride Injection, USP prior to use |
6 |
0.3 mL |
2.1 Preparation for Administration
Prior to Dilution
- The Pfizer-BioNTech COVID-19 Vaccine multiple dose vial with a purple cap contains a volume of 0.45 mL and is supplied as a frozen suspension that does not contain preservative.
- Each vial must be thawed before dilution.
- Vials may be thawed in the refrigerator [2ºC to 8ºC (35ºF to 46ºF)] or at room temperature [up to 25ºC (77ºF)] [see How Supplied/Storage and Handling (19)].
- Refer to thawing instructions in the panels below.
Dilution
- Dilute the vial contents using 1.8 mL of sterile 0.9% Sodium Chloride Injection, USP (not provided) to form the Pfizer-BioNTech COVID-19 Vaccine. Do not add more than 1.8 mL of diluent.
- ONLY use sterile 0.9% Sodium Chloride Injection, USP as the diluent. This diluent is not packaged with the vaccine and must be sourced separately. Do not use bacteriostatic 0.9% Sodium Chloride Injection or any other diluent.
- After dilution, 1 vial contains 6 doses of 0.3 mL.
2.2 Administration Information
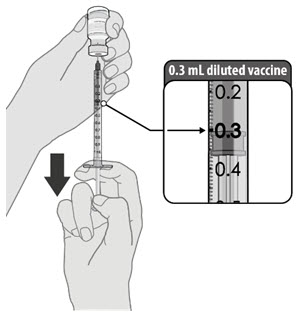
Visually inspect each dose in the dosing syringe prior to administration. The vaccine will be an off-white suspension. During the visual inspection,
- verify the final dosing volume of 0.3 mL.
- confirm there are no particulates and that no discoloration is observed.
- do not administer if vaccine is discolored or contains particulate matter.
Administer the Pfizer-BioNTech COVID-19 Vaccine intramuscularly.
After dilution, vials of Pfizer-BioNTech COVID-19 Vaccine with purple caps contain 6 doses of 0.3 mL of vaccine. Low dead-volume syringes and/or needles can be used to extract 6 doses from a single vial. If standard syringes and needles are used, there may not be sufficient volume to extract 6 doses from a single vial. Irrespective of the type of syringe and needle:
- Each dose must contain 0.3 mL of vaccine.
- If the amount of vaccine remaining in the vial cannot provide a full dose of 0.3 mL, discard the vial and any excess volume.
- Do not pool excess vaccine from multiple vials.
2.3 Vaccination Schedule
Primary Series5
The Pfizer-BioNTech COVID-19 Vaccine is administered intramuscularly as a primary series of 2 doses (0.3 mL each) 3 weeks apart in individuals 12 years of age and older.
A third primary series dose of the Pfizer-BioNTech COVID-19 Vaccine (0.3 mL) at least 28 days following the second dose is authorized for administration to individuals at least 12 years of age with certain kinds of immunocompromise.6
3 DOSAGE FORMS AND STRENGTHS
Pfizer-BioNTech COVID-19 Vaccine is a suspension for injection.
After preparation, each dose of the Pfizer-BioNTech COVID-19 Vaccine supplied in vials with purple caps is 0.3 mL for individuals 12 years of age and older [see Dosage and Administration (2.1)].
4 CONTRAINDICATIONS
Do not administer Pfizer-BioNTech COVID-19 Vaccine to individuals with known history of a severe allergic reaction (e.g., anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 Vaccine [see Description (13)].
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of Pfizer-BioNTech COVID-19 Vaccine.
Monitor Pfizer-BioNTech COVID-19 Vaccine recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention (CDC) guidelines (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
5.2 Myocarditis and Pericarditis
Postmarketing data with Pfizer-BioNTech COVID-19 Vaccine demonstrate increased risks of myocarditis and pericarditis, particularly within the period 0 through 7 days following the second dose of the primary series. The observed risk is higher among adolescent males and adult males under 40 years of age than among females and older males. The observed risk is highest in males 12 through 17 years of age. Although some cases required intensive care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae. The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html).
5.3 Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines, in particular in adolescents. Procedures should be in place to avoid injury from fainting.
6 OVERALL SAFETY SUMMARY
It is MANDATORY for vaccination providers to report to the Vaccine Adverse Event Reporting System (VAERS) all vaccine administration errors, all serious adverse events, cases of myocarditis, cases of pericarditis, cases of Multisystem Inflammatory Syndrome (MIS) in adults and children, and hospitalized or fatal cases of COVID-19 following vaccination with the Pfizer-BioNTech COVID-19 Vaccine.7 To the extent feasible, provide a copy of the VAERS form to Pfizer Inc. Please see the REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS section for details on reporting to VAERS and Pfizer Inc.
Primary Series
In clinical studies of participants 16 years of age and older who received Pfizer-BioNTech COVID-19 Vaccine containing 30 mcg of a nucleoside-modified messenger RNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 (30 mcg modRNA), adverse reactions following administration of the primary series included pain at the injection site (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%), injection site swelling (10.5%), injection site redness (9.5%), nausea (1.1%), malaise (0.5%), and lymphadenopathy (0.3%).
In a clinical study in adolescents 12 through 15 years of age who received Pfizer-BioNTech COVID-19 Vaccine (30 mcg modRNA), adverse reactions following administration of the primary series included pain at the injection site (90.5%), fatigue (77.5%), headache (75.5%), chills (49.2%), muscle pain (42.2%), fever (24.3%), joint pain (20.2%), injection site swelling (9.2%), injection site redness (8.6%), lymphadenopathy (0.8%), and nausea (0.4%).
Post Authorization Experience
Severe allergic reactions, including anaphylaxis, have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine.
Myocarditis and pericarditis have been reported following administration of the Pfizer-BioNTech COVID-19 Vaccine.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Primary Series
The safety of the primary series Pfizer-BioNTech COVID-19 Vaccine was evaluated in participants 12 years of age and older in two clinical studies conducted in the United States, Europe, Turkey, South Africa, and South America.
Study BNT162-01 (Study 1) was a Phase 1/2, 2-part, dose-escalation trial that enrolled 60 participants, 18 through 55 years of age. Study C4591001 (Study 2) is a Phase 1/2/3, multicenter, multinational, randomized, saline placebo-controlled, observer-blind, dose-finding, vaccine candidate-selection (Phase 1) and efficacy (Phase 2/3) study that has enrolled approximately 46,000 participants, 12 years of age or older. Of these, approximately 43,448 participants [21,720 Pfizer-BioNTech COVID-19 Vaccine (30 mcg modRNA) encoding the viral spike (S) glycoprotein of SARS-CoV-2; 21,728 placebo] in Phase 2/3 are 16 years of age or older (including 138 and 145 adolescents 16 and 17 years of age in the vaccine and placebo groups, respectively) and 2,260 adolescents are 12 through 15 years of age (1,131 and 1,129 in the vaccine and placebo groups, respectively).
In Study 2, all participants 12 through 15 years of age, and 16 years of age and older in the reactogenicity subset, were monitored for solicited local and systemic reactions and use of antipyretic medication after each vaccination in an electronic diary. Participants are being monitored for unsolicited adverse events, including serious adverse events, throughout the study [from Dose 1 through 1 month (all unsolicited adverse events) or 6 months (serious adverse events) after the last vaccination]. Tables 1 through 6 present the frequency and severity of solicited local and systemic reactions, respectively, within 7 days following each dose of Pfizer-BioNTech COVID 19 Vaccine and placebo.
Participants 16 Years of Age and Older
At the time of the analysis of Study 2 for the EUA, 37,586 [18,801 Pfizer-BioNTech COVID-19 Vaccine (30 mcg modRNA) and 18,785 placebo] participants 16 years of age or older had been followed for a median of 2 months after the second dose.
The safety evaluation in Study 2 is ongoing. The safety population includes participants 16 years of age and older enrolled by October 9, 2020, and includes safety data accrued through November 14, 2020.
Demographic characteristics in Study 2 were generally similar with regard to age, gender, race, and ethnicity among participants who received Pfizer-BioNTech COVID-19 Vaccine and those who received placebo. Overall, among the total participants who received either the Pfizer-BioNTech COVID-19 Vaccine or placebo, 50.6% were male and 49.4% were female, 83.1% were White, 9.1% were Black or African American, 28.0% were Hispanic/Latino, 4.3% were Asian, and 0.5% were American Indian/Alaska Native.
Solicited Local and Systemic Adverse Reactions
Across both age groups, 18 through 55 years of age and 56 years of age and older, the mean duration of pain at the injection site after Dose 2 was 2.5 days (range 1 to 36 days), for redness 2.6 days (range 1 to 34 days), and for swelling 2.3 days (range 1 to 34 days) for participants in the Pfizer-BioNTech COVID-19 Vaccine group.
Solicited reactogenicity data in 16 and 17 year-old participants are limited.
| Note: Reactions were collected in the electronic diary (e-diary) from Day 1 to Day 7 after vaccination. | ||||
|
|
||||
|
Redness# |
||||
|
Any (>2 cm) |
104 (4.5) |
26 (1.1) |
123 (5.9) |
14 (0.7) |
|
Mild |
70 (3.1) |
16 (0.7) |
73 (3.5) |
8 (0.4) |
|
Moderate |
28 (1.2) |
6 (0.3) |
40 (1.9) |
6 (0.3) |
|
Severe |
6 (0.3) |
4 (0.2) |
10 (0.5) |
0 (0.0) |
|
Swelling# |
||||
|
Any (>2 cm) |
132 (5.8) |
11 (0.5) |
132 (6.3) |
5 (0.2) |
|
Mild |
88 (3.8) |
3 (0.1) |
80 (3.8) |
3 (0.1) |
|
Moderate |
39 (1.7) |
5 (0.2) |
45 (2.1) |
2 (0.1) |
|
Severe |
5 (0.2) |
3 (0.1) |
7 (0.3) |
0 (0.0) |
|
Pain at the injection siteÞ |
||||
|
Any |
1904 (83.1) |
322 (14.0) |
1632 (77.8) |
245 (11.7) |
|
Mild |
1170 (51.1) |
308 (13.4) |
1039 (49.5) |
225 (10.7) |
|
Moderate |
710 (31.0) |
12 (0.5) |
568 (27.1) |
20 (1.0) |
|
Severe |
24 (1.0) |
2 (0.1) |
25 (1.2) |
0 (0.0) |
| Pfizer-BioNTech COVID-19 Vaccine‡
Dose 1 N§=2291 n¶ (%) | Placebo
Dose 1 N§=2298 n¶ (%) | Pfizer-BioNTech COVID-19 Vaccine‡
Dose 2 N§=2098 n¶ (%) | Placebo
Dose 2 N§=2103 n¶ (%) |
|
|---|---|---|---|---|
| Note: Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary) from Day 1 to Day 7 after each dose. | ||||
|
|
||||
|
Fever |
||||
|
≥38.0°C |
85 (3.7) |
20 (0.9) |
331 (15.8) |
10 (0.5) |
|
≥38.0°C to 38.4°C |
64 (2.8) |
10 (0.4) |
194 (9.2) |
5 (0.2) |
|
>38.4°C to 38.9°C |
15 (0.7) |
5 (0.2) |
110 (5.2) |
3 (0.1) |
|
>38.9°C to 40.0°C |
6 (0.3) |
3 (0.1) |
26 (1.2) |
2 (0.1) |
|
>40.0°C |
0 (0.0) |
2 (0.1) |
1 (0.0) |
0 (0.0) |
|
Fatigue# | ||||
|
Any |
1085 (47.4) |
767 (33.4) |
1247 (59.4) |
479 (22.8) |
|
Mild |
597 (26.1) |
467 (20.3) |
442 (21.1) |
248 (11.8) |
|
Moderate |
455 (19.9) |
289 (12.6) |
708 (33.7) |
217 (10.3) |
|
Severe |
33 (1.4) |
11 (0.5) |
97 (4.6) |
14 (0.7) |
|
Headache# | ||||
|
Any |
959 (41.9) |
775 (33.7) |
1085 (51.7) |
506 (24.1) |
|
Mild |
628 (27.4) |
505 (22.0) |
538 (25.6) |
321 (15.3) |
|
Moderate |
308 (13.4) |
251 (10.9) |
480 (22.9) |
170 (8.1) |
|
Severe |
23 (1.0) |
19 (0.8) |
67 (3.2) |
15 (0.7) |
|
Chills# | ||||
|
Any |
321 (14.0) |
146 (6.4) |
737 (35.1) |
79 (3.8) |
|
Mild |
230 (10.0) |
111 (4.8) |
359 (17.1) |
65 (3.1) |
|
Moderate |
82 (3.6) |
33 (1.4) |
333 (15.9) |
14 (0.7) |
|
Severe |
9 (0.4) |
2 (0.1) |
45 (2.1) |
0 (0.0) |
|
VomitingÞ | ||||
|
Any |
28 (1.2) |
28 (1.2) |
40 (1.9) |
25 (1.2) |
|
Mild |
24 (1.0) |
22 (1.0) |
28 (1.3) |
16 (0.8) |
|
Moderate |
4 (0.2) |
5 (0.2) |
8 (0.4) |
9 (0.4) |
|
Severe |
0 (0.0) |
1 (0.0) |
4 (0.2) |
0 (0.0) |
|
Diarrheaß | ||||
|
Any |
255 (11.1) |
270 (11.7) |
219 (10.4) |
177 (8.4) |
|
Mild |
206 (9.0) |
217 (9.4) |
179 (8.5) |
144 (6.8) |
|
Moderate |
46 (2.0) |
52 (2.3) |
36 (1.7) |
32 (1.5) |
|
Severe |
3 (0.1) |
1 (0.0) |
4 (0.2) |
1 (0.0) |
|
New or worsened muscle pain# |
||||
|
Any |
487 (21.3) |
249 (10.8) |
783 (37.3) |
173 (8.2) |
|
Mild |
256 (11.2) |
175 (7.6) |
326 (15.5) |
111 (5.3) |
|
Moderate |
218 (9.5) |
72 (3.1) |
410 (19.5) |
59 (2.8) |
|
Severe |
13 (0.6) |
2 (0.1) |
47 (2.2) |
3 (0.1) |
|
New or worsened joint pain# |
||||
|
Any |
251 (11.0) |
138 (6.0) |
459 (21.9) |
109 (5.2) |
|
Mild |
147 (6.4) |
95 (4.1) |
205 (9.8) |
54 (2.6) |
|
Moderate |
99 (4.3) |
43 (1.9) |
234 (11.2) |
51 (2.4) |
|
Severe |
5 (0.2) |
0 (0.0) |
20 (1.0) |
4 (0.2) |
|
Use of antipyretic or pain medicationà |
638 (27.8) |
332 (14.4) |
945 (45.0) |
266 (12.6) |
| Pfizer-BioNTech COVID-19 Vaccine†
Dose 1 N‡=1802 n§ (%) | Placebo
Dose 1 N‡=1792 n§ (%) | Pfizer-BioNTech COVID-19 Vaccine†
Dose 2 N‡=1660 n§ (%) | Placebo
Dose 2 N‡=1646 n§ (%) |
|
|---|---|---|---|---|
| Note: Reactions were collected in the electronic diary (e-diary) from Day 1 to Day 7 after vaccination. | ||||
|
|
||||
|
Redness¶ | ||||
|
Any (>2 cm) |
85 (4.7) |
19 (1.1) |
120 (7.2) |
12 (0.7) |
|
Mild |
55 (3.1) |
12 (0.7) |
59 (3.6) |
8 (0.5) |
|
Moderate |
27 (1.5) |
5 (0.3) |
53 (3.2) |
3 (0.2) |
|
Severe |
3 (0.2) |
2 (0.1) |
8 (0.5) |
1 (0.1) |
|
Swelling¶ | ||||
|
Any (>2 cm) |
118 (6.5) |
21 (1.2) |
124 (7.5) |
11 (0.7) |
|
Mild |
71 (3.9) |
10 (0.6) |
68 (4.1) |
5 (0.3) |
|
Moderate |
45 (2.5) |
11 (0.6) |
53 (3.2) |
5 (0.3) |
|
Severe |
2 (0.1) |
0 (0.0) |
3 (0.2) |
1 (0.1) |
|
Pain at the injection site# | ||||
|
Any (>2 cm) |
1282 (71.1) |
166 (9.3) |
1098 (66.1) |
127 (7.7) |
|
Mild |
1008 (55.9) |
160 (8.9) |
792 (47.7) |
125 (7.6) |
|
Moderate |
270 (15.0) |
6 (0.3) |
298 (18.0) |
2 (0.1) |
|
Severe |
4 (0.2) |
0 (0.0) |
8 (0.5) |
0 (0.0) |
| Pfizer-BioNTech COVID-19 Vaccine†
Dose 1 N‡=1802 n§ (%) | Placebo
Dose 1 N‡=1792 n§ (%) | Pfizer-BioNTech COVID-19 Vaccine†
Dose 2 N‡=1660 n§ (%) | Placebo
Dose 2 N‡=1646 n§ (%) |
|
|---|---|---|---|---|
| Note: Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary) from Day 1 to Day 7 after each dose. | ||||
|
|
||||
|
Fever | ||||
|
≥38.0°C |
26 (1.4) |
7 (0.4) |
181 (10.9) |
4 (0.2) |
|
≥38.0°C to 38.4°C |
23 (1.3) |
2 (0.1) |
131 (7.9) |
2 (0.1) |
|
>38.4°C to 38.9°C |
1 (0.1) |
3 (0.2) |
45 (2.7) |
1 (0.1) |
|
>38.9°C to 40.0°C |
1 (0.1) |
2 (0.1) |
5 (0.3) |
1 (0.1) |
|
>40.0°C |
1 (0.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Fatigue¶ | ||||
|
Any |
615 (34.1) |
405 (22.6) |
839 (50.5) |
277 (16.8) |
|
Mild |
373 (20.7) |
252 (14.1) |
351 (21.1) |
161 (9.8) |
|
Moderate |
240 (13.3) |
150 (8.4) |
442 (26.6) |
114 (6.9) |
|
Severe |
2 (0.1) |
3 (0.2) |
46 (2.8) |
2 (0.1) |
|
Headache¶ | ||||
|
Any |
454 (25.2) |
325 (18.1) |
647 (39.0) |
229 (13.9) |
|
Mild |
348 (19.3) |
242 (13.5) |
422 (25.4) |
165 (10.0) |
|
Moderate |
104 (5.8) |
80 (4.5) |
216 (13.0) |
60 (3.6) |
|
Severe |
2 (0.1) |
3 (0.2) |
9 (0.5) |
4 (0.2) |
|
Chills¶ | ||||
|
Any |
113 (6.3) |
57 (3.2) |
377 (22.7) |
46 (2.8) |
|
Mild |
87 (4.8) |
40 (2.2) |
199 (12.0) |
35 (2.1) |
|
Moderate |
26 (1.4) |
16 (0.9) |
161 (9.7) |
11 (0.7) |
|
Severe |
0 (0.0) |
1 (0.1) |
17 (1.0) |
0 (0.0) |
|
Vomiting# | ||||
|
Any |
9 (0.5) |
9 (0.5) |
11 (0.7) |
5 (0.3) |
|
Mild |
8 (0.4) |
9 (0.5) |
9 (0.5) |
5 (0.3) |
|
Moderate |
1 (0.1) |
0 (0.0) |
1 (0.1) |
0 (0.0) |
|
Severe |
0 (0.0) |
0 (0.0) |
1 (0.1) |
0 (0.0) |
|
DiarrheaÞ | ||||
|
Any |
147 (8.2) |
118 (6.6) |
137 (8.3) |
99 (6.0) |
|
Mild |
118 (6.5) |
100 (5.6) |
114 (6.9) |
73 (4.4) |
|
Moderate |
26 (1.4) |
17 (0.9) |
21 (1.3) |
22 (1.3) |
|
Severe |
3 (0.2) |
1 (0.1) |
2 (0.1) |
4 (0.2) |
|
New or worsened muscle pain¶ |
||||
|
Any |
251 (13.9) |
149 (8.3) |
477 (28.7) |
87 (5.3) |
|
Mild |
168 (9.3) |
100 (5.6) |
202 (12.2) |
57 (3.5) |
|
Moderate |
82 (4.6) |
46 (2.6) |
259 (15.6) |
29 (1.8) |
|
Severe |
1 (0.1) |
3 (0.2) |
16 (1.0) |
1 (0.1) |
|
New or worsened joint pain¶ |
||||
|
Any |
155 (8.6) |
109 (6.1) |
313 (18.9) |
61 (3.7) |
|
Mild |
101 (5.6) |
68 (3.8) |
161 (9.7) |
35 (2.1) |
|
Moderate |
52 (2.9) |
40 (2.2) |
145 (8.7) |
25 (1.5) |
|
Severe |
2 (0.1) |
1 (0.1) |
7 (0.4) |
1 (0.1) |
|
Use of antipyretic or pain medication |
358 (19.9) |
213 (11.9) |
625 (37.7) |
161 (9.8) |
From an independent report (Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med), in 99 individuals who had undergone various solid organ transplant procedures (heart, kidney, liver, lung, pancreas) 97±8 months previously who received a third vaccine dose, the adverse event profile was similar to that after the second dose and no grade 3 or grade 4 events were reported in recipients who were followed for 1 month following post Dose 3.
Unsolicited Adverse Events
Serious Adverse Events
In Study 2, among participants 16 through 55 years of age who had received at least 1 dose of vaccine or placebo (Pfizer-BioNTech COVID-19 Vaccine = 10,841; placebo = 10,851), serious adverse events from Dose 1 through up to 30 days after Dose 2 in ongoing follow-up were reported by 0.4% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 0.3% of placebo recipients. In a similar analysis, in participants 56 years of age and older (Pfizer-BioNTech COVID-19 Vaccine = 7,960, placebo = 7,934), serious adverse events were reported by 0.8% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 0.6% of placebo recipients who received at least 1 dose of Pfizer-BioNTech COVID-19 Vaccine or placebo, respectively. In these analyses, 91.6% of study participants had at least 30 days of follow-up after Dose 2.
Appendicitis was reported as a serious adverse event for 12 participants, and numerically higher in the vaccine group, 8 vaccine participants and 4 placebo participants. Currently available information is insufficient to determine a causal relationship with the vaccine. There were no other notable patterns or numerical imbalances between treatment groups for specific categories of serious adverse events (including neurologic, neuro-inflammatory, and thrombotic events) that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
Non-Serious Adverse Events
In Study 2 in which 10,841 participants 16 through 55 years of age received Pfizer-BioNTech COVID-19 Vaccine and 10,851 participants received placebo, non-serious adverse events from Dose 1 through up to 30 days after Dose 2 in ongoing follow-up were reported in 29.3% of participants who received Pfizer-BioNTech COVID-19 Vaccine and 13.2% of participants in the placebo group, for participants who received at least 1 dose. Overall in a similar analysis in which 7960 participants 56 years of age and older received Pfizer-BioNTech COVID-19 Vaccine, non-serious adverse events within 30 days were reported in 23.8% of participants who received Pfizer-BioNTech COVID-19 Vaccine and 11.7% of participants in the placebo group, for participants who received at least 1 dose. In these analyses, 91.6% of study participants had at least 30 days of follow-up after Dose 2.
The higher frequency of reported unsolicited non-serious adverse events among Pfizer-BioNTech COVID-19 Vaccine recipients compared to placebo recipients was primarily attributed to local and systemic adverse events reported during the first 7 days following vaccination that are consistent with adverse reactions solicited among participants in the reactogenicity subset and presented in Tables 3 and 4. From Dose 1 through 30 days after Dose 2, reports of lymphadenopathy were imbalanced with notably more cases in the Pfizer-BioNTech COVID-19 Vaccine group (64) vs. the placebo group (6), which is plausibly related to vaccination. Throughout the safety follow-up period to date, Bell's palsy (facial paralysis) was reported by 4 participants in the Pfizer-BioNTech COVID-19 Vaccine group. Onset of facial paralysis was Day 37 after Dose 1 (participant did not receive Dose 2) and Days 3, 9, and 48 after Dose 2. No cases of Bell's palsy were reported in the placebo group. Currently available information is insufficient to determine a causal relationship with the vaccine. There were no other notable patterns or numerical imbalances between treatment groups for specific categories of non-serious adverse events (including other neurologic or neuro-inflammatory, and thrombotic events) that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
Adolescents 12 Through 15 Years of Age
In an analysis of Study 2, based on data up to the cutoff date of March 13, 2021, 2,260 adolescents (1,131 Pfizer-BioNTech COVID-19 Vaccine (30 mcg modRNA); 1,129 placebo) were 12 through 15 years of age. Of these, 1,308 (660 Pfizer-BioNTech COVID-19 Vaccine and 648 placebo) adolescents have been followed for at least 2 months after the second dose. The safety evaluation in Study 2 is ongoing.
Demographic characteristics in Study 2 were generally similar with regard to age, gender, race, and ethnicity among adolescents who received Pfizer-BioNTech COVID-19 Vaccine and those who received placebo. Overall, among the adolescents who received the Pfizer-BioNTech COVID-19 Vaccine, 50.1% were male and 49.9% were female, 85.9% were White, 4.6% were Black or African American, 11.7% were Hispanic/Latino, 6.4% were Asian, and 0.4% were American Indian/Alaska Native.
Solicited Local and Systemic Adverse Reactions
The mean duration of pain at the injection site after Dose 1 was 2.4 days (range 1 to 10 days), for redness 2.4 days (range 1 to 16 days), and for swelling 1.9 days (range 1 to 5 days) for adolescents in the Pfizer-BioNTech COVID-19 Vaccine group.
| Pfizer-BioNTech COVID-19 Vaccine†
Dose 1 N‡=1127 n§ (%) | Placebo
Dose 1 N‡=1127 n§ (%) | Pfizer-BioNTech COVID-19 Vaccine†
Dose 2 N‡=1097 n§ (%) | Placebo
Dose 2 N‡=1078 n§ (%) |
|
|---|---|---|---|---|
| Note: Reactions were collected in the electronic diary (e-diary) from Day 1 to Day 7 after vaccination. | ||||
|
|
||||
|
Redness¶ | ||||
|
Any (>2 cm) |
65 (5.8) |
12 (1.1) |
55 (5.0) |
10 (0.9) |
|
Mild |
44 (3.9) |
11 (1.0) |
29 (2.6) |
8 (0.7) |
|
Moderate |
20 (1.8) |
1 (0.1) |
26 (2.4) |
2 (0.2) |
|
Severe |
1 (0.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Swelling¶ | ||||
|
Any (>2 cm) |
78 (6.9) |
11 (1.0) |
54 (4.9) |
6 (0.6) |
|
Mild |
55 (4.9) |
9 (0.8) |
36 (3.3) |
4 (0.4) |
|
Moderate |
23 (2.0) |
2 (0.2) |
18 (1.6) |
2 (0.2) |
|
Severe |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Pain at the injection site# | ||||
|
Any |
971 (86.2) |
263 (23.3) |
866 (78.9) |
193 (17.9) |
|
Mild |
467 (41.4) |
227 (20.1) |
466 (42.5) |
164 (15.2) |
|
Moderate |
493 (43.7) |
36 (3.2) |
393 (35.8) |
29 (2.7) |
|
Severe |
11 (1.0) |
0 (0.0) |
7 (0.6) |
0 (0.0) |
| Pfizer-BioNTech COVID-19 Vaccine†
Dose 1 N‡=1127 n§ (%) | Placebo
Dose 1 N‡=1127 n§ (%) | Pfizer-BioNTech COVID-19 Vaccine†
Dose 2 N‡=1097 n§ (%) | Placebo
Dose 2 N‡=1078 n§ (%) |
|
|---|---|---|---|---|
| Note: Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary) from Day 1 to Day 7 after each dose. | ||||
|
|
||||
|
Fever | ||||
|
≥38.0°C |
114 (10.1) |
12 (1.1) |
215 (19.6) |
7 (0.6) |
|
≥38.0°C to 38.4°C |
74 (6.6) |
8 (0.7) |
107 (9.8) |
5 (0.5) |
|
>38.4°C to 38.9°C |
29 (2.6) |
2 (0.2) |
83 (7.6) |
1 (0.1) |
|
>38.9°C to 40.0°C |
10 (0.9) |
2 (0.2) |
25 (2.3) |
1 (0.1) |
|
>40.0°C |
1 (0.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Fatigue¶ | ||||
|
Any |
677 (60.1) |
457 (40.6) |
726 (66.2) |
264 (24.5) |
|
Mild |
278 (24.7) |
250 (22.2) |
232 (21.1) |
133 (12.3) |
|
Moderate |
384 (34.1) |
199 (17.7) |
468 (42.7) |
127 (11.8) |
|
Severe |
15 (1.3) |
8 (0.7) |
26 (2.4) |
4 (0.4) |
|
Headache¶ | ||||
|
Any |
623 (55.3) |
396 (35.1) |
708 (64.5) |
263 (24.4) |
|
Mild |
361 (32.0) |
256 (22.7) |
302 (27.5) |
169 (15.7) |
|
Moderate |
251 (22.3) |
131 (11.6) |
384 (35.0) |
93 (8.6) |
|
Severe |
11 (1.0) |
9 (0.8) |
22 (2.0) |
1 (0.1) |
|
Chills¶ | ||||
|
Any |
311 (27.6) |
109 (9.7) |
455 (41.5) |
73 (6.8) |
|
Mild |
195 (17.3) |
82 (7.3) |
221 (20.1) |
52 (4.8) |
|
Moderate |
111 (9.8) |
25 (2.2) |
214 (19.5) |
21 (1.9) |
|
Severe |
5 (0.4) |
2 (0.2) |
20 (1.8) |
0 (0.0) |
|
Vomiting# | ||||
|
Any |
31 (2.8) |
10 (0.9) |
29 (2.6) |
12 (1.1) |
|
Mild |
30 (2.7) |
8 (0.7) |
25 (2.3) |
11 (1.0) |
|
Moderate |
0 (0.0) |
2 (0.2) |
4 (0.4) |
1 (0.1) |
|
Severe |
1 (0.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
DiarrheaÞ | ||||
|
Any |
90 (8.0) |
82 (7.3) |
65 (5.9) |
43 (4.0) |
|
Mild |
77 (6.8) |
72 (6.4) |
59 (5.4) |
38 (3.5) |
|
Moderate |
13 (1.2) |
10 (0.9) |
6 (0.5) |
5 (0.5) |
|
Severe |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
New or worsened muscle pain¶ |
||||
|
Any |
272 (24.1) |
148 (13.1) |
355 (32.4) |
90 (8.3) |
|
Mild |
125 (11.1) |
88 (7.8) |
152 (13.9) |
51 (4.7) |
|
Moderate |
145 (12.9) |
60 (5.3) |
197 (18.0) |
37 (3.4) |
|
Severe |
2 (0.2) |
0 (0.0) |
6 (0.5) |
2 (0.2) |
|
New or worsened joint pain¶ |
||||
|
Any |
109 (9.7) |
77 (6.8) |
173 (15.8) |
51 (4.7) |
|
Mild |
66 (5.9) |
50 (4.4) |
91 (8.3) |
30 (2.8) |
|
Moderate |
42 (3.7) |
27 (2.4) |
78 (7.1) |
21 (1.9) |
|
Severe |
1 (0.1) |
0 (0.0) |
4 (0.4) |
0 (0.0) |
|
Use of antipyretic or pain medicationß |
413 (36.6) |
111 (9.8) |
557 (50.8) |
95 (8.8) |
Unsolicited Adverse Events
In the following analyses of Study 2 in adolescents 12 through 15 years of age (1,131 of whom received Pfizer-BioNTech COVID-19 Vaccine and 1,129 of whom received placebo), 98.3% of study participants had at least 30 days of follow-up after Dose 2.
Serious Adverse Events
Serious adverse events from Dose 1 through up to 30 days after Dose 2 in ongoing follow-up were reported by 0.4% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 0.1% of placebo recipients. There were no notable patterns or numerical imbalances between treatment groups for specific categories of serious adverse events that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
Non-Serious Adverse Events
Non-serious adverse events from Dose 1 through up to 30 days after Dose 2 in ongoing follow-up were reported by 5.8% of Pfizer-BioNTech COVID-19 Vaccine recipients and by 5.8% of placebo recipients. From Dose 1 through 30 days after Dose 2, reports of lymphadenopathy plausibly related to the study intervention were imbalanced, with notably more cases in the Pfizer-BioNTech COVID-19 Vaccine group (7) vs. the placebo group (1). There were no other notable patterns or numerical imbalances between treatment groups for specific categories of non-serious adverse events that would suggest a causal relationship to Pfizer-BioNTech COVID-19 Vaccine.
6.2 Post Authorization Experience
The following adverse reactions have been identified during post authorization use of Pfizer-BioNTech COVID-19 Vaccine. Because these reactions are reported voluntarily, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Cardiac Disorders: myocarditis, pericarditis
Gastrointestinal Disorders: diarrhea, vomiting
Immune System Disorders: severe allergic reactions, including anaphylaxis, and other hypersensitivity reactions (e.g., rash, pruritus, urticaria, angioedema)
Musculoskeletal and Connective Tissue Disorders: pain in extremity (arm)
Nervous System Disorders: syncope, dizziness
8 REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS8
See Overall Safety Summary (Section 6) for additional information.
The vaccination provider enrolled in the federal COVID-19 Vaccination Program is responsible for MANDATORY reporting of the listed events following Pfizer-BioNTech COVID-19 Vaccine to the Vaccine Adverse Event Reporting System (VAERS):
- Vaccine administration errors whether or not associated with an adverse event
- Serious adverse events* (irrespective of attribution to vaccination)
- Cases of myocarditis
- Cases of pericarditis
- Cases of Multisystem Inflammatory Syndrome (MIS) in children and adults
- Cases of COVID-19 that result in hospitalization or death
*Serious adverse events are defined as:
- Death
- A life-threatening adverse event
- Inpatient hospitalization or prolongation of existing hospitalization
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions
- A congenital anomaly/birth defect
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent 1 of the outcomes listed above
Instructions for Reporting to VAERS
The vaccination provider enrolled in the federal COVID-19 Vaccination Program should complete and submit a VAERS form to FDA using 1 of the following methods:
- Complete and submit the report online: https://vaers.hhs.gov/reportevent.html, or
- If you are unable to submit this form electronically, you may fax it to VAERS at 1-877-721-0366. If you need additional help submitting a report you may call the VAERS toll-free information line at 1-800-822-7967 or send an email to info@vaers.org.
IMPORTANT: When reporting adverse events or vaccine administration errors to VAERS, please complete the entire form with detailed information. It is important that the information reported to FDA be as detailed and complete as possible. Information to include:
- Patient demographics (e.g., patient name, date of birth)
- Pertinent medical history
- Pertinent details regarding admission and course of illness
- Concomitant medications
- Timing of adverse event(s) in relationship to administration of the Pfizer-BioNTech COVID-19 Vaccine
- Pertinent laboratory and virology information
- Outcome of the event and any additional follow-up information if it is available at the time of the VAERS report. Subsequent reporting of follow-up information should be completed if additional details become available.
The following steps are highlighted to provide the necessary information for safety tracking:
- 1. In Box 17, provide information on Pfizer-BioNTech COVID-19 Vaccine and any other vaccines administered on the same day; and in Box 22, provide information on any other vaccines received within 1 month prior.
- 2.
In Box 18, description of the event:
- a. Write "Pfizer-BioNTech COVID-19 Vaccine EUA" as the first line.
- b. Provide a detailed report of vaccine administration error and/or adverse event. It is important to provide detailed information regarding the patient and adverse event/medication error for ongoing safety evaluation of this unapproved vaccine. Please see information to include listed above.
- 3.
Contact information:
- a. In Box 13, provide the name and contact information of the prescribing healthcare provider or institutional designee who is responsible for the report.
- b. In Box 14, provide the name and contact information of the best doctor/healthcare professional to contact about the adverse event.
- c. In Box 15, provide the address of the facility where vaccine was given (NOT the healthcare provider's office address).
Other Reporting Instructions
Vaccination providers may report to VAERS other adverse events that are not required to be reported using the contact information above.
To the extent feasible, report adverse events to Pfizer Inc. using the contact information below or by providing a copy of the VAERS form to Pfizer Inc.
| Website | Fax number | Telephone number |
|---|---|---|
|
1-866-635-8337 |
1-800-438-1985 |
10 DRUG INTERACTIONS
There are no data to assess the concomitant administration of the Pfizer-BioNTech COVID-19 Vaccine with other vaccines.
11 USE IN SPECIFIC POPULATIONS
11.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. Available data on Pfizer-BioNTech COVID-19 Vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
In a reproductive and developmental toxicity study, 0.06 mL of a vaccine formulation containing the same quantity of nucleoside-modified messenger ribonucleic acid (modRNA) (30 mcg) and other ingredients included in a single human dose of Pfizer-BioNTech COVID-19 Vaccine was administered to female rats by the intramuscular route on 4 occasions: 21 and 14 days prior to mating, and on gestation days 9 and 20. No vaccine-related adverse effects on female fertility, fetal development, or postnatal development were reported in the study.
11.3 Pediatric Use
Pfizer-BioNTech COVID-19 Vaccine is authorized for use in individuals 6 months through 17 years of age. This authorization is based on safety and effectiveness data in this age group and adults.
Pfizer-BioNTech COVID-19 Vaccine is not authorized for use in individuals younger than 6 months of age.
11.4 Geriatric Use
Clinical studies of Pfizer-BioNTech COVID-19 Vaccine include participants 65 years of age and older who received the primary series and their data contributes to the overall assessment of safety and efficacy [see Overall Safety Summary (6.1) and Clinical Trial Results and Supporting Data for EUA (18.1)]. Of the total number of Pfizer-BioNTech COVID-19 Vaccine recipients in Study 2 (N=20,033), 21.4% (n=4,294) were 65 years of age and older and 4.3% (n=860) were 75 years of age and older.
11.5 Use in Immunocompromised
From an independent report (Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med), safety and effectiveness of a third dose of the Pfizer-BioNTech COVID-19 vaccine have been evaluated in persons that received solid organ transplants. The administration of a third dose of vaccine appears to be only moderately effective in increasing potentially protective antibody titers. Patients should still be counselled to maintain physical precautions to help prevent COVID-19. In addition, close contacts of immunocompromised persons should be vaccinated as appropriate for their health status.
13 DESCRIPTION
The Pfizer-BioNTech COVID-19 Vaccine is supplied as a frozen suspension in multiple dose vials with purple caps; each vial must be diluted with 1.8 mL of sterile 0.9% Sodium Chloride Injection, USP prior to use to form the vaccine. Each 0.3 mL dose of the Pfizer-BioNTech COVID-19 Vaccine supplied in multiple dose vials with purple caps contains 30 mcg of a nucleoside-modified messenger RNA (modRNA) encoding the viral spike (S) glycoprotein of the SARS-CoV-2 Wuhan-Hu-1 strain.
Each 0.3 mL dose of the Pfizer-BioNTech COVID-19 Vaccine supplied in multiple dose vials with purple caps also includes the following ingredients: lipids (0.43 mg ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 0.05 mg 2[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 0.09 mg 1,2-distearoyl-sn-glycero-3-phosphocholine, and 0.2 mg cholesterol), 0.01 mg potassium chloride, 0.01 mg monobasic potassium phosphate, 0.36 mg sodium chloride, 0.07 mg dibasic sodium phosphate dihydrate, and 6 mg sucrose. The diluent (sterile 0.9% Sodium Chloride Injection, USP) contributes an additional 2.16 mg sodium chloride per dose.
The Pfizer-BioNTech COVID-19 Vaccine does not contain preservative. The vial stoppers are not made with natural rubber latex.
18 CLINICAL TRIAL RESULTS AND SUPPORTING DATA FOR EUA
18.1 Efficacy of Primary Series in Participants 16 Years of Age and Older
Study 2 is a multicenter, multinational, Phase 1/2/3, randomized, placebo-controlled, observer-blind, dose-finding, vaccine candidate-selection, and efficacy study in participants 12 years of age and older. Randomization was stratified by age: 12 through 15 years of age, 16 through 55 years of age, or 56 years of age and older, with a minimum of 40% of participants in the ≥56-year stratum. The study excluded participants who were immunocompromised and those who had previous clinical or microbiological diagnosis of COVID-19. Participants with preexisting stable disease, defined as disease not requiring significant change in therapy or hospitalization for worsening disease during the 6 weeks before enrollment, were included as were participants with known stable infection with human immunodeficiency virus (HIV), hepatitis C virus (HCV), or hepatitis B virus (HBV).
In the Phase 2/3 portion of Study 2, based on data accrued through November 14, 2020, approximately 44,000 participants 12 years of age and older were randomized equally and received 2 doses of Pfizer-BioNTech COVID-19 Vaccine (30 mcg modRNA) or placebo separated by 21 days. Participants are planned to be followed for up to 24 months, for assessments of safety and efficacy against COVID-19.
The population for the analysis of the primary efficacy endpoint included, 36,621 participants 12 years of age and older (18,242 in the Pfizer-BioNTech COVID-19 Vaccine group and 18,379 in the placebo group) who did not have evidence of prior infection with SARS-CoV-2 through 7 days after the second dose. Table 7 presents the specific demographic characteristics in the studied population.
|
Chronic lung disease (e.g., emphysema and chronic bronchitis, idiopathic pulmonary fibrosis, and cystic fibrosis) or moderate to severe asthma Significant cardiac disease (e.g., heart failure, coronary artery disease, congenital heart disease, cardiomyopathies, and pulmonary hypertension) Obesity (body mass index ≥30 kg/m2) Diabetes (Type 1, Type 2 or gestational) Liver disease |
||
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Placebo
|
|
|
Sex |
||
|
Male |
9318 (51.1) |
9225 (50.2) |
|
Female |
8924 (48.9) |
9154 (49.8) |
|
Age (years) |
||
|
Mean (SD) |
50.6 (15.70) |
50.4 (15.81) |
|
Median |
52.0 |
52.0 |
|
Min, max |
(12, 89) |
(12, 91) |
|
Age group |
||
|
≥12 through 15 years‡ |
46 (0.3) |
42 (0.2) |
|
≥16 through 17 years |
66 (0.4) |
68 (0.4) |
|
≥16 through 64 years |
14,216 (77.9) |
14,299 (77.8) |
|
≥65 through 74 years |
3176 (17.4) |
3226 (17.6) |
|
≥75 years |
804 (4.4) |
812 (4.4) |
|
Race |
||
|
White |
15,110 (82.8) |
15,301 (83.3) |
|
Black or African American |
1617 (8.9) |
1617 (8.8) |
|
American Indian or Alaska Native |
118 (0.6) |
106 (0.6) |
|
Asian |
815 (4.5) |
810 (4.4) |
|
Native Hawaiian or other Pacific Islander |
48 (0.3) |
29 (0.2) |
|
Other§ |
534 (2.9) |
516 (2.8) |
|
Ethnicity |
||
|
Hispanic or Latino |
4886 (26.8) |
4857 (26.4) |
|
Not Hispanic or Latino |
13,253 (72.7) |
13,412 (73.0) |
|
Not reported |
103 (0.6) |
110 (0.6) |
|
Comorbidities¶ |
||
|
Yes |
8432 (46.2) |
8450 (46.0) |
|
No |
9810 (53.8) |
9929 (54.0) |
Human Immunodeficiency Virus (HIV) infection (not included in the efficacy evaluation)
The population in the primary efficacy analysis included all participants 12 years of age and older who had been enrolled from July 27, 2020, and followed for the development of COVID-19 through November 14, 2020. Participants 18 through 55 years of age and 56 years of age and older began enrollment from July 27, 2020, 16 through 17 years of age began enrollment from September 16, 2020, and 12 through 15 years of age began enrollment from October 15, 2020.
The vaccine efficacy information is presented in Table 8.
| Note: Confirmed cases were determined by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and at least 1 symptom consistent with COVID-19 (symptoms included: fever; new or increased cough; new or increased shortness of breath; chills; new or increased muscle pain; new loss of taste or smell; sore throat; diarrhea; vomiting). | |||
|
|
|||
|
First COVID-19 occurrence from 7 days after Dose 2 in participants without evidence of prior SARS-CoV-2 infection* |
|||
|
Subgroup |
Pfizer-BioNTech COVID-19 Vaccine†
|
Vaccine Efficacy %
|
|
|
All subjectsÞ |
8 |
162 |
95.0 |
|
16 through 64 years |
7 |
143 |
95.1 |
|
65 years and older |
1 |
19 |
94.7 |
|
First COVID-19 occurrence from 7 days after Dose 2 in participants with or without evidence of prior SARS-CoV-2 infection |
|||
|
Subgroup |
Pfizer-BioNTech COVID-19 Vaccine†
|
Vaccine Efficacy %
|
|
|
All subjectsÞ |
9 |
169 |
94.6 |
|
16 through 64 years |
8 |
150 |
94.6 |
|
65 years and older |
1 |
19 |
94.7 |
18.2 Efficacy of Primary Series in Adolescents 12 Through 15 Years of Age
A descriptive efficacy analysis of Study 2 has been performed in approximately 2,200 adolescents 12 through 15 years of age evaluating confirmed COVID-19 cases accrued up to a data cutoff date of March 13, 2021.
The efficacy information in adolescents 12 through 15 years of age is presented in Table 9.
| Note: Confirmed cases were determined by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and at least 1 symptom consistent with COVID-19 (symptoms included: fever; new or increased cough; new or increased shortness of breath; chills; new or increased muscle pain; new loss of taste or smell; sore throat; diarrhea; vomiting). | |||
|
|
|||
|
First COVID-19 occurrence from 7 days after Dose 2 in adolescents 12 through 15 years of age without evidence of prior SARS-CoV-2 infection* |
|||
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Vaccine Efficacy %
|
||
|
Adolescents |
0 |
16 |
100.0 |
|
First COVID-19 occurrence from 7 days after Dose 2 in adolescents 12 through 15 years of age with or without evidence of prior SARS-CoV-2 infection |
|||
|
Pfizer-BioNTech COVID-19 Vaccine†
|
Vaccine Efficacy %
|
||
|
Adolescents |
0 |
18 |
100.0 |
18.3 Immunogenicity of Primary Series in Adolescents 12 Through 15 Years of Age
In Study 2, an analysis of SARS-CoV-2 50% neutralizing titers (NT50) 1 month after Dose 2 in a randomly selected subset of participants demonstrated non-inferior immune responses (within 1.5-fold) comparing adolescents 12 through 15 years of age to participants 16 through 25 years of age who had no serological or virological evidence of past SARS-CoV-2 infection up to 1 month after Dose 2 (Table 10).
| Pfizer-BioNTech COVID-19 Vaccine* | |||||
|---|---|---|---|---|---|
| 12 Through 15 Years
n†=190 | 16 Through 25 Years
n†=170 | 12 Through 15 Years/16 Through 25 Years | |||
| Assay | Time Point‡ | GMT§
(95% CI§) | GMT§
(95% CI§) | GMR¶
(95% CI¶) | Met Noninferiority Objective#
(Y/N) |
| Abbreviations: CI = confidence interval; GMR = geometric mean ratio; GMT = geometric mean titer; LLOQ = lower limit of quantitation; NAAT = nucleic-acid amplification test; NT50 = 50% neutralizing titer; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. Note: Participants who had no serological or virological evidence (up to 1 month after receipt of the last dose) of past SARS-CoV-2 infection (i.e., N-binding antibody [serum] negative at Visit 1 and SARS-CoV-2 not detected by NAAT [nasal swab] at Visits 1 and 2), and had negative NAAT (nasal swab) at any unscheduled visit up to 1 month after Dose 2 were included in the analysis. |
|||||
|
|
|||||
|
SARS-CoV-2 neutralization assay - NT50 (titer)Þ |
1 month after Dose 2 |
1239.5 |
705.1 |
1.76 |
Y |
18.4 Immunogenicity of a Third Primary Series Dose in Individuals with Certain Kinds of Immunocompromise
From an independent report (Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med), a single arm study has been conducted in 101 individuals who had undergone various solid organ transplant procedures (heart, kidney, liver, lung, pancreas) 97±8 months previously. A third dose of the Pfizer-BioNTech COVID-19 vaccine was administered to 99 of these individuals approximately 2 months after they had received a second dose. Among the 59 patients who had been seronegative before the third dose, 26 (44%) were seropositive at 4 weeks after the third dose. All 40 patients who had been seropositive before the third dose were still seropositive 4 weeks later. The prevalence of anti-SARS-CoV-2 antibodies was 68% (67 of 99 patients) 4 weeks after the third dose.
19 HOW SUPPLIED/STORAGE AND HANDLING
The information in this section applies to the Pfizer-BioNTech COVID-19 Vaccine that is supplied in multiple dose vials with a purple cap. These multiple dose vials are supplied in a carton containing 25 multiple dose vials (NDC: 59267-1000-3) or 195 multiple dose vials (NDC: 59267-1000-2). After dilution, 1 vial contains 6 doses of 0.3 mL. Vial labels and cartons may state that after dilution, a vial contains 5 doses of 0.3 mL. The information in this Full EUA Prescribing Information regarding the number of doses per vial after dilution supersedes the number of doses stated on vial labels and cartons.
During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
Do not refreeze thawed vials.
Frozen Vials Prior to Use
Cartons of Pfizer-BioNTech COVID-19 Vaccine multiple dose vials with purple caps arrive in thermal containers with dry ice. Once received, remove the vial cartons immediately from the thermal container and preferably store in an ultra-low temperature freezer between -90ºC to -60ºC (-130ºF to -76ºF) until the expiry date printed on the label. This information in the package insert supersedes the storage conditions printed on the vial cartons.
Cartons and vials of Pfizer-BioNTech COVID-19 Vaccine supplied in multiple dose vials with purple caps with an expiry date of December 2021 through December 2022 printed on the label may remain in use beyond the printed date until the updated expiry date shown below; as long as approved storage conditions have been maintained.
|
Printed Expiry Date |
Updated Expiry Date |
|
|
12/2021 |
➜ |
31-Dec-2022 |
|
01/2022 |
➜ |
31-Jan-2023 |
|
02/2022 |
➜ |
28-Feb-2023 |
|
03/2022 |
➜ |
31-Mar-2023 |
|
06/2022 |
➜ |
31-Mar-2023 |
|
07/2022 |
➜ |
30-Apr-2023 |
|
08/2022 |
➜ |
31-May-2023 |
|
09/2022 |
➜ |
30-Jun-2023 |
|
10/2022 |
➜ |
31-Jul-2023 |
|
11/2022 |
➜ |
31-Aug-2023 |
|
12/2022 |
➜ |
30-Sep-2023 |
If not stored between -90ºC to -60ºC (-130ºF to -76ºF), vials may be stored at -25°C to -15°C (-13°F to 5°F) for up to 2 weeks. Vials must be kept frozen and protected from light, in the original cartons, until ready to use. Vials stored at -25°C to -15°C (-13°F to 5°F) for up to 2 weeks may be returned one time to the recommended storage condition of -90ºC to -60ºC (-130ºF to -76ºF). Total cumulative time the vials are stored at -25°C to -15°C (-13°F to 5°F) should be tracked and should not exceed 2 weeks.
If an ultra-low temperature freezer is not available, the thermal container in which the Pfizer-BioNTech COVID-19 Vaccine arrives may be used as temporary storage when consistently re-filled to the top of the container with dry ice. Refer to the re-icing guidelines packed in the original thermal container for instructions regarding the use of the thermal container for temporary storage. The thermal container maintains a temperature range of -90ºC to -60ºC (-130ºF to -76ºF). Storage of the vials between -96°C to -60°C (-141°F to -76°F) is not considered an excursion from the recommended storage condition.
Transportation of Frozen Vials
If local redistribution is needed and full cartons containing vials cannot be transported at -90°C to -60°C (-130°F to -76°F), vials may be transported at -25°C to -15°C (-13°F to 5°F). Any hours used for transport at -25°C to -15°C (-13°F to 5°F) count against the 2-week limit for storage at -25°C to -15°C (-13°F to 5°F). Frozen vials transported at -25°C to -15°C (-13°F to 5°F) may be returned one time to the recommended storage condition of -90ºC to -60ºC (-130ºF to -76ºF).
Thawed Vials Before Dilution
Thawed Under Refrigeration
Thaw and then store undiluted vials in the refrigerator [2ºC to 8ºC (35ºF to 46ºF)] for up to 1 month. A carton of 25 vials or 195 vials may take up to 2 or 3 hours, respectively, to thaw in the refrigerator, whereas a fewer number of vials will thaw in less time.
Thawed at Room Temperature
For immediate use, thaw undiluted vials at room temperature [up to 25ºC (77ºF)] for 30 minutes. Thawed vials can be handled in room light conditions.
Vials must reach room temperature before dilution.
Undiluted vials may be stored at room temperature for no more than 2 hours.
Transportation of Thawed Vials
Available data support transportation of one or more thawed vials at 2°C to 8°C (35°F to 46°F) for up to 48 hours.
Vials After Dilution
After dilution, store vials between 2°C to 25°C (35°F to 77°F) and use within 6 hours from the time of dilution. During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light. Any vaccine remaining in vials must be discarded after 6 hours. Do not refreeze.
20 PATIENT COUNSELING INFORMATION
Advise the recipient or caregiver to read the Vaccine Information Fact Sheet for Recipients and Caregivers.
The vaccination provider must include vaccination information in the state/local jurisdiction's Immunization Information System (IIS) or other designated system. Advise recipient or caregiver that more information about IISs can be found at: https://www.cdc.gov/vaccines/programs/iis/about.html.
21 CONTACT INFORMATION
For general questions, visit the website or call the telephone number provided below.
| Website | Telephone number |
|---|---|
|
1-877-829-2619 |
This Full EUA Prescribing Information may have been updated. For the most recent Full EUA Prescribing Information, please see www.cvdvaccine.com.
Manufactured for
BioNTech Manufacturing GmbH
An der Goldgrube 12
55131 Mainz, Germany
Manufactured by
Pfizer Inc., New York, NY 10017
LAB-1457-30.0
Revised: 22 December 2022
VACCINE INFORMATION FACT SHEET FOR RECIPIENTS AND CAREGIVERS ABOUT COMIRNATY (COVID-19 VACCINE, mRNA), THE PFIZER-BIONTECH COVID-19 VACCINE, AND THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT (ORIGINAL AND OMICRON BA.4/BA.5) TO PREVENT CORONAVIRUS DISEASE 2019 (COVID-19) FOR USE IN INDIVIDUALS 12 YEARS OF AGE AND OLDER
|
FOR 12 YEARS OF AGE AND OLDER |
You are being offered either COMIRNATY (COVID-19 Vaccine, mRNA), the Pfizer-BioNTech COVID-19 Vaccine, or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), hereafter referred to as the Pfizer-BioNTech COVID-19 Vaccine, Bivalent, to prevent Coronavirus Disease 2019 (COVID-19) caused by SARS-CoV-2.
This Vaccine Information Fact Sheet for Recipients and Caregivers comprises the Fact Sheet for the authorized Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent, and also includes information about the U.S. Food and Drug Administration (FDA)-licensed vaccine, COMIRNATY (COVID-19 Vaccine, mRNA) for use in individuals 12 years of age and older9.
The FDA-approved COMIRNATY (COVID-19 Vaccine, mRNA) and the Pfizer-BioNTech COVID-19 Vaccine authorized under Emergency Use Authorization (EUA) for individuals 12 years of age and older, when prepared according to their respective instructions for use, can be used interchangeably.10
COMIRNATY (COVID-19 Vaccine, mRNA) is an FDA-approved COVID-19 vaccine made by Pfizer for BioNTech. It is approved as a 2-dose series for prevention of COVID-19 in individuals 12 years of age and older. It is also authorized under EUA to provide:
- a third primary series dose to individuals 12 years of age and older with certain kinds of immunocompromise.
The Pfizer-BioNTech COVID-19 Vaccine has received EUA from FDA to provide:
- a 2-dose primary series to individuals 12 years of age and older; and
- a third primary series dose to individuals 12 years of age and older with certain kinds of immunocompromise.
The Pfizer-BioNTech COVID-19 Vaccine, Bivalent has received EUA from FDA to provide either:
- a single booster dose to individuals 12 years of age and older at least 2 months after completion of primary vaccination with any authorized or approved COVID-19 vaccine; or
- a single booster dose to individuals 12 years of age and older at least 2 months after receipt of the most recent booster dose with any authorized or approved monovalent11 COVID-19 vaccine.
This Vaccine Information Fact Sheet contains information to help you understand the risks and benefits of COMIRNATY (COVID-19 Vaccine, mRNA), the Pfizer-BioNTech COVID-19 Vaccine, and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent, which you may receive because there is currently a pandemic of COVID-19. Talk to your vaccination provider if you have questions.
This Fact Sheet may have been updated. For the most recent Fact Sheet, please see www.cvdvaccine.com.
WHAT YOU NEED TO KNOW BEFORE YOU GET ANY OF THESE VACCINES
WHAT IS COVID-19?
COVID-19 disease is caused by a coronavirus called SARS-CoV-2. You can get COVID-19 through contact with another person who has the virus. It is predominantly a respiratory illness that can affect other organs. People with COVID-19 have had a wide range of symptoms reported, ranging from mild symptoms to severe illness leading to death. Symptoms may appear 2 to 14 days after exposure to the virus. Symptoms may include: fever or chills; cough; shortness of breath; fatigue; muscle or body aches; headache; new loss of taste or smell; sore throat; congestion or runny nose; nausea or vomiting; diarrhea.
HOW ARE COMIRNATY (COVID-19 VACCINE, mRNA), THE PFIZER-BIONTECH COVID-19 VACCINE, AND THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT RELATED?
COMIRNATY (COVID-19 Vaccine, mRNA) and the Pfizer-BioNTech COVID-19 Vaccine, when prepared according to their respective instructions for use, can be used interchangeably. The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is made in the same way as COMIRNATY and Pfizer-BioNTech COVID-19 Vaccine but it also contains an Omicron component to help prevent COVID-19 caused by the Omicron variant of SARS-CoV-2.
For more information on EUA, see the "What is an Emergency Use Authorization (EUA)?" section at the end of this Fact Sheet.
WHAT SHOULD YOU MENTION TO YOUR VACCINATION PROVIDER BEFORE YOU GET ANY OF THESE VACCINES?
Tell the vaccination provider about all of your medical conditions, including if you:
- have any allergies
- have had myocarditis (inflammation of the heart muscle) or pericarditis (inflammation of the lining outside the heart)
- have a fever
- have a bleeding disorder or are on a blood thinner
- are immunocompromised or are on a medicine that affects your immune system
- are pregnant or plan to become pregnant
- are breastfeeding
- have received another COVID-19 vaccine
- have ever fainted in association with an injection
HOW ARE THESE VACCINES GIVEN?
The Pfizer-BioNTech COVID-19 Vaccine, the Pfizer-BioNTech COVID-19 Vaccine, Bivalent, or COMIRNATY (COVID-19 Vaccine, mRNA) will be given to you as an injection into the muscle.
Primary Series: The Pfizer-BioNTech COVID-19 Vaccine and COMIRNATY (COVID-19 Vaccine, mRNA) are given for the primary series. The vaccine is administered as a 2-dose series, 3 weeks apart. A third primary series dose may be administered at least 4 weeks after the second dose to individuals with certain kinds of immunocompromise.
Booster Dose: Pfizer-BioNTech COVID-19 Vaccine, Bivalent is administered as a single booster dose at least 2 months after:
- completion of primary vaccination with any authorized or approved COVID-19 vaccine; or
- receipt of the most recent booster dose with any authorized or approved monovalent COVID-19 vaccine
The vaccine may not protect everyone.
WHO SHOULD NOT GET COMIRNATY (COVID-19 VACCINE, mRNA), THE PFIZER-BIONTECH COVID-19 VACCINE, OR THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT?
You should not get any of these vaccines if you:
- had a severe allergic reaction after a previous dose of COMIRNATY (COVID-19 Vaccine, mRNA) or the Pfizer-BioNTech COVID-19 Vaccine
- had a severe allergic reaction to any ingredient in these vaccines.
WHAT ARE THE INGREDIENTS IN THESE VACCINES?
COMIRNATY (COVID-19 Vaccine, mRNA), Pfizer-BioNTech COVID-19 Vaccine, and Pfizer-BioNTech COVID-19 Vaccine, Bivalent include the following ingredients:
- mRNA and lipids (((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-Distearoyl-sn-glycero-3-phosphocholine, and cholesterol).
Pfizer-BioNTech COVID-19 Vaccine for individuals 12 years of age and older contains 1 of the following sets of additional ingredients; ask the vaccination provider which version is being administered:
- potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose
OR
- tromethamine, tromethamine hydrochloride, and sucrose
Pfizer-BioNTech COVID-19 Vaccine, Bivalent for individuals 12 years of age and older contains the following additional ingredients:
- tromethamine, tromethamine hydrochloride, and sucrose
COMIRNATY (COVID-19 Vaccine, mRNA) contains 1 of the following sets of additional ingredients; ask the vaccination provider which version is being administered:
- potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose
OR
- tromethamine, tromethamine hydrochloride, and sucrose
HAVE THESE VACCINES BEEN USED BEFORE?
In clinical trials, approximately 23,000 individuals 12 years of age and older have received at least 1 dose of Pfizer-BioNTech COVID-19 Vaccine. Millions of individuals have received the Pfizer-BioNTech COVID-19 Vaccine under EUA since December 11, 2020.
In a clinical trial, approximately 300 individuals greater than 55 years of age received one dose of a bivalent vaccine that differs from the Pfizer-BioNTech COVID-19 Vaccine, Bivalent in that it contains a different Omicron component.
WHAT ARE THE BENEFITS OF THESE VACCINES?
COMIRNATY (COVID-19 Vaccine, mRNA) and the Pfizer-BioNTech COVID-19 Vaccine have been shown to prevent COVID-19. FDA has authorized Pfizer-BioNTech COVID-19 Vaccine, Bivalent to provide better protection against COVID-19 caused by the Omicron variant of SARS-CoV-2.
The duration of protection against COVID-19 is currently unknown.
WHAT ARE THE RISKS OF THESE VACCINES?
There is a remote chance that these vaccines could cause a severe allergic reaction. A severe allergic reaction would usually occur within a few minutes to 1 hour after getting a dose. For this reason, your vaccination provider may ask you to stay at the place where you received your vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include:
- Difficulty breathing
- Swelling of your face and throat
- A fast heartbeat
- A bad rash all over your body
- Dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received COMIRNATY (COVID-19 Vaccine, mRNA) or Pfizer-BioNTech COVID-19 Vaccine, more commonly in adolescent males and adult males under 40 years of age than among females and older males. In most of these people, symptoms began within a few days following receipt of the second dose of vaccine. The chance of having this occur is very low. You should seek medical attention right away if you have any of the following symptoms after receiving the vaccine:
- Chest pain
- Shortness of breath
- Feelings of having a fast-beating, fluttering, or pounding heart
Side effects that have been reported with these vaccines include:
- Severe allergic reactions
- Non-severe allergic reactions such as rash, itching, hives, or swelling of the face
- Myocarditis (inflammation of the heart muscle)
- Pericarditis (inflammation of the lining outside the heart)
- Injection site pain
- Tiredness
- Headache
- Muscle pain
- Chills
- Joint pain
- Fever
- Injection site swelling
- Injection site redness
- Nausea
- Feeling unwell
- Swollen lymph nodes (lymphadenopathy)
- Decreased appetite
- Diarrhea
- Vomiting
- Arm pain
- Fainting in association with injection of the vaccine
- Dizziness
These may not be all the possible side effects of these vaccines. Serious and unexpected side effects may occur. The possible side effects of these vaccines are still being studied.
WHAT SHOULD I DO ABOUT SIDE EFFECTS?
If you experience a severe allergic reaction, call 9-1-1, or go to the nearest hospital.
Call the vaccination provider or your healthcare provider if you have any side effects that bother you or do not go away.
Report vaccine side effects to FDA/CDC Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to https://vaers.hhs.gov/reportevent.html. Please include either "COMIRNATY (COVID-19 Vaccine, mRNA)", "Pfizer-BioNTech COVID-19 Vaccine EUA", or "Pfizer-BioNTech COVID-19 Vaccine, Bivalent EUA" as appropriate, in the first line of box #18 of the report form.
In addition, you can report side effects to Pfizer Inc. at the contact information provided below.
| Website | Fax number | Telephone number |
|---|---|---|
|
1-866-635-8337 |
1-800-438-1985 |
You may also be given an option to enroll in v-safe. V-safe is a voluntary smartphone-based tool that uses text messaging and web surveys to check in with people who have been vaccinated to identify potential side effects after COVID-19 vaccination. V-safe asks questions that help CDC monitor the safety of COVID-19 vaccines. V-safe also provides second-dose reminders if needed and live telephone follow-up by CDC if participants report a significant health impact following COVID-19 vaccination. For more information on how to sign up, visit: www.cdc.gov/vsafe.
WHAT IF I DECIDE NOT TO GET COMIRNATY (COVID-19 VACCINE, mRNA), THE PFIZER-BIONTECH COVID-19 VACCINE, OR THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT?
Under the EUA, it is your choice to receive or not receive any of these vaccines. Should you decide not to receive any of these vaccines, it will not change your standard medical care.
ARE OTHER CHOICES AVAILABLE FOR PREVENTING COVID-19 BESIDES COMIRNATY (COVID-19 VACCINE, mRNA), THE PFIZER-BIONTECH COVID-19 VACCINE, OR THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT?
For primary vaccination, another choice for preventing COVID-19 is SPIKEVAX (COVID-19 Vaccine, mRNA), an FDA-approved COVID-19 vaccine. Other vaccines to prevent COVID-19 may be available under EUA, including bivalent vaccines that contain an Omicron component of SARS-CoV-2.
CAN I RECEIVE COMIRNATY (COVID-19 VACCINE, mRNA), PFIZER-BIONTECH COVID-19 VACCINE, OR THE PFIZER-BIONTECH COVID-19 VACCINE, BIVALENT AT THE SAME TIME AS OTHER VACCINES?
Data have not yet been submitted to FDA on administration of COMIRNATY (COVID-19 Vaccine, mRNA), the Pfizer-BioNTech COVID-19 Vaccine, or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent at the same time with other vaccines. If you are considering receiving COMIRNATY (COVID-19 Vaccine, mRNA), the Pfizer-BioNTech COVID-19 Vaccine, or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent with other vaccines, discuss your options with your healthcare provider.
WHAT IF I AM IMMUNOCOMPROMISED?
If you are immunocompromised, you may receive a third primary series dose of Pfizer-BioNTech COVID-19 Vaccine or COMIRNATY (COVID-19 Vaccine, mRNA). Individuals 12 years of age and older may receive a booster dose with Pfizer-BioNTech COVID-19 Vaccine, Bivalent. Vaccinations may not provide full immunity to COVID-19 in people who are immunocompromised, and you should continue to maintain physical precautions to help prevent COVID-19. Your close contacts should be vaccinated as appropriate.
WHAT IF I AM PREGNANT OR BREASTFEEDING?
If you are pregnant or breastfeeding, discuss your options with your healthcare provider.
WILL THESE VACCINES GIVE ME COVID-19?
No. These vaccines do not contain SARS-CoV-2 and cannot give you COVID-19.
KEEP YOUR VACCINATION CARD
When you get your first COVID-19 vaccine, you will get a vaccination card. Remember to bring your card when you return.
ADDITIONAL INFORMATION
If you have questions, visit the website or call the telephone number provided below.
To access the most recent Fact Sheets, please scan the QR code provided below.
| Global website | Telephone number |
|---|---|
|
1-877-829-2619 |
HOW CAN I LEARN MORE?
- Ask the vaccination provider.
- Visit CDC at https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- Visit FDA at https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
- Contact your local or state public health department.
WHERE WILL MY VACCINATION INFORMATION BE RECORDED?
The vaccination provider may include your vaccination information in your state/local jurisdiction's Immunization Information System (IIS) or other designated system. For more information about IISs visit: https://www.cdc.gov/vaccines/programs/iis/about.html.
CAN I BE CHARGED AN ADMINISTRATION FEE FOR RECEIPT OF THESE COVID-19 VACCINES?
No. At this time, the provider cannot charge you for a vaccine dose and you cannot be charged an out-of-pocket vaccine administration fee or any other fee if only receiving a COVID-19 vaccination. However, vaccination providers may seek appropriate reimbursement from a program or plan that covers COVID-19 vaccine administration fees for the vaccine recipient (private insurance, Medicare, Medicaid, Health Resources & Services Administration [HRSA] COVID-19 Uninsured Program for non-insured recipients).
WHERE CAN I REPORT CASES OF SUSPECTED FRAUD?
Individuals becoming aware of any potential violations of the CDC COVID-19 Vaccination Program requirements are encouraged to report them to the Office of the Inspector General, U.S. Department of Health and Human Services, at 1-800-HHS-TIPS or https://TIPS.HHS.GOV.
WHAT IS THE COUNTERMEASURES INJURY COMPENSATION PROGRAM?
The Countermeasures Injury Compensation Program (CICP) is a federal program that may help pay for costs of medical care and other specific expenses of certain people who have been seriously injured by certain medicines or vaccines, including these vaccines. Generally, a claim must be submitted to the CICP within one (1) year from the date of receiving the vaccine. To learn more about this program, visit http://www.hrsa.gov/cicp/ or call 1-855-266-2427.
WHAT IS AN EMERGENCY USE AUTHORIZATION (EUA)?
An EUA is a mechanism to facilitate the availability and use of medical products, including vaccines, during public health emergencies, such as the current COVID-19 pandemic. An EUA is supported by a Secretary of Health and Human Services (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic. A product authorized for emergency use has not undergone the same type of review by FDA as an FDA-approved product.
FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, and available alternatives. In addition, the FDA decision is based on the totality of the scientific evidence available showing that the product may be effective to prevent COVID-19 during the COVID-19 pandemic and that the known and potential benefits of the product outweigh the known and potential risks of the product. All of these criteria must be met to allow for the product to be used during the COVID-19 pandemic.
An EUA is in effect for the duration of the COVID-19 EUA declaration justifying emergency use of this product, unless terminated or revoked (after which the product may no longer be used).
Manufactured for
BioNTech Manufacturing GmbH
An der Goldgrube 12
55131 Mainz, Germany

Manufactured by
Pfizer Inc., New York, NY 10017
LAB-1451-24.0
Revised: 8 December 2022
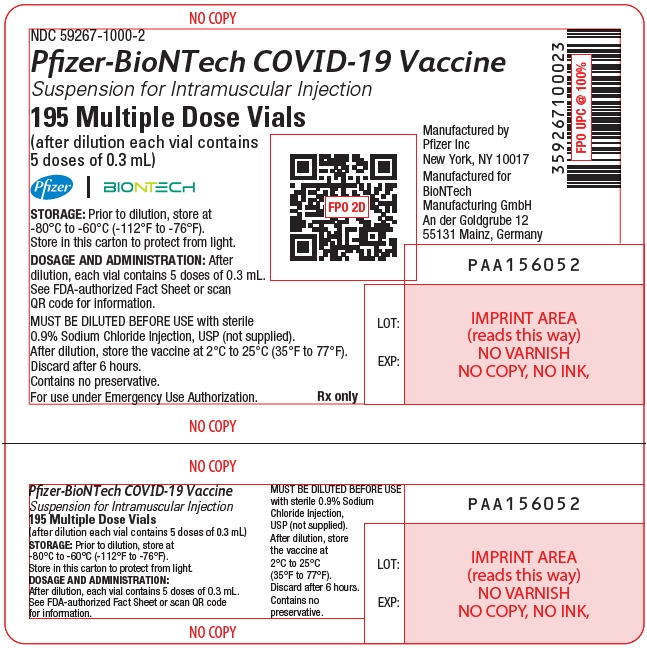 |
Scan to capture that this Fact Sheet was provided to vaccine recipient for the electronic medical records/immunization information systems. |
|
GDTI: 0886983000332 |
|
PRINCIPAL DISPLAY PANEL - 1.8 mL Vial Label
Pfizer-BioNTech COVID-19 Vaccine
After dilution, vial contains 6 doses of 0.3 mL
For intramuscular use. Contains no preservative.
For use under Emergency Use Authorization.
DILUTE BEFORE USE. Discard 6 hours after
dilution when stored at 2 to 25°C (35 to 77°F).
Dilution date and time:
NDC: 59267-1000-1
PRINCIPAL DISPLAY PANEL - 195 Vial Carton Label
NDC: 59267-1000-2
Pfizer-BioNTech COVID-19 Vaccine
Suspension for Intramuscular Injection
195 Multiple Dose Vials
(after dilution each vial contains
6 doses of 0.3 mL)
Pfizer BIONTECH
STORAGE: Prior to dilution, store at
-80°C to -60°C (-112°F to -76°F).
Store in this carton to protect from light.
DOSAGE AND ADMINISTRATION: After
dilution, each vial contains 6 doses of 0.3 mL.
See FDA-authorized Fact Sheet or scan
QR code for information.
MUST BE DILUTED BEFORE USE with sterile
0.9% Sodium Chloride Injection, USP (not supplied).
After dilution, store the vaccine at 2°C to 25°C (35°F to 77°F).
Discard after 6 hours.
Contains no preservative.
For use under Emergency Use Authorization.
Rx only
Manufactured by
Pfizer Inc
New York, NY 10017
Manufactured for
BioNTech
Manufacturing GmbH
An der Goldgrube 12
55131 Mainz, Germany
PAA166261
LOT:
EXP:
PRINCIPAL DISPLAY PANEL - 25 Vial Carton
NDC: 59267-1000-3
Rx only
Pfizer-BioNTech COVID-19 Vaccine
Suspension for Intramuscular Injection
25 Multiple Dose Vials
(after dilution each vial contains 6 doses of 0.3 mL)
MUST BE DILUTED BEFORE USE with sterile
0.9% Sodium Chloride Injection, USP (not supplied).
After dilution, store the vaccine at 2°C to 25°C (35°F to 77°F).
Discard after 6 hours.
| PFIZER-BIONTECH COVID-19 VACCINE
bnt162b2 injection, suspension |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Pfizer Manufacturing Belgium NV (370156507) |
| Registrant - Pfizer Inc (113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Belgium NV | 370156507 | ANALYSIS(59267-1000) , MANUFACTURE(59267-1000) , PACK(59267-1000) , LABEL(59267-1000) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(59267-1000) , MANUFACTURE(59267-1000) , PACK(59267-1000) , LABEL(59267-1000) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC | 174350868 | ANALYSIS(59267-1000) , API MANUFACTURE(59267-1000) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Inc | 004954111 | ANALYSIS(59267-1000) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Exela Pharma Sciences, LLC. | 831274399 | ANALYSIS(59267-1000) , MANUFACTURE(59267-1000) , PACK(59267-1000) , LABEL(59267-1000) | |