INDOCYANINE GREEN- indocyanine green and water kit
Indocyanine Green by
Drug Labeling and Warnings
Indocyanine Green by is a Prescription medication manufactured, distributed, or labeled by Olympus America, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Indocyanine Green for Injection USP safely and effectively. See full prescribing information for Indocyanine Green for Injection USP.
Indocyanine Green for Injection USP Fore Intravenous Injection
Initial U.S. Approval: 1959INDICATIONS AND USAGE
Indocyanine Green for Injection USP a tricarbocyanine dye, is indicated:
- For visual assessment of blood vessels, blood flow and related tissue perfusion with OLYMPUS infrared compatible endoscopic imaging system (1.1)
- For visual assessment of the major extrahepatic bile ducts with OLYMPUS infrared imaging endoscopic imaging system (1.2)
- For viewing intra-operative blood flow in the cerebral area with OLYMPUS infrared compatible video microscopic imaging system (1.3)
DOSAGE AND ADMINISTRATION
For visual assessment of blood vessels, blood flow and related tissue perfusion (2.1)
Under sterile conditions, the Indocyanine Green for Injection USP powder should be dissolved with the 10 mL Sterile Water for Injection, USP provided and the solution used within 6 hours after it is prepared. The usual doses of Indocyanine Green for Injection USP is 0.1 mg/kg (0.04 mL/kg) – 0.3 mg/kg (0.12 mL/kg). Immediately follow each ICG intravenous injection with a tight bolus injection of approximately 10 - 12 mL of normal saline immediately before IR imaging. Multiple administrations can be performed, up to 2 mg/kg (0.8 mL/kg) per patient.
For visual assessment of the major extrahepatic bile ducts (2.2)
Under sterile conditions, the Indocyanine Green for Injection USP powder should be dissolved with the 10 mL Sterile Water for Injection, USP provided and the solution used within 6 hours after it is prepared. The usual doses of Indocyanine Green for Injection USP is 3.0 mg (1.2 mL). Immediately follow each ICG intravenous injection with a tight bolus injection of approximately 10 - 12 mL of normal saline at least 30 minutes prior to IR imaging. Multiple administrations can be performed, up to 2 mg/kg (0.8 mL/kg) per patient.
For viewing intra-operative blood flow in the cerebral area (2.3)
Under sterile conditions, the Indocyanine Green for Injection USP powder should be dissolved with the 10 mL Sterile Water for Injection, USP provided and the solution used within 6 hours after it is prepared. The usual doses of Indocyanine Green for Injection USP is 0.1 mg/kg (0.04 mL/kg) – 0.3 mg/kg (0.12 mL/kg). Immediately follow each ICG intravenous injection with a tight bolus injection of approximately 10 - 12 mL of normal saline immediately before IR imaging Multiple administrations can be performed, up to 2 mg/kg (0.8 mL/kg) per patient.
DOSAGE FORMS AND STRENGTHS
Indocyanine Green for Injection USP is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5% sodium iodide. (3)
CONTRAINDICATIONS
Indocyanine Green for Injection USP contains sodium iodide and should be used with caution in patients who have a history of allergy to iodides because of the risk of anaphylaxis. (4)
WARNINGS AND PRECAUTIONS
- Deaths due to anaphylaxis have been reported following Indocyanine Green for Injection USP administration during cardiac catheterization. (5.1)
- Indocyanine Green for Injection USP is unstable in aqueous solution and must be used within 6 hours. (5.2)
- Radioactive iodine uptake studies should not be performed for at least a week following the use of Indocyanine Green for Injection USP. (5.3)
ADVERSE REACTIONS
Most common adverse reactions are anaphylactic or urticarial reactions. These have been reported in patients with and without a history of allergy to iodides. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Diagnostic Green LLC at 1-844-424-3784 (1-844-ICG-DRUG) or e-mail: drugsafety@ diagnosticgreen.com; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Products containing sodium bisulfite reduce the absorption peak of Indocyanine Green for Injection USP in blood. (7)
Revised: 6/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Visual assessment of blood vessels, blood flow and related tissue perfusion
1.2 Visual assessment of the major extrahepatic bile duct
1.3 Viewing intra-operative blood flow in the cerebral area
2 DOSAGE AND ADMINISTRATION
2.1 Visual assessment of blood vessels, blood flow and related tissue perfusion
2.2 Visual assessment of the major extrahepatic bile duct
2.3 Viewing intra-operative blood flow in the cerebral area
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis
5.2 Drug Instability
5.3 Drug/Laboratory Test Interactions
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Indocyanine Green for Injection USP is indicated:
1.1 Visual assessment of blood vessels, blood flow and related tissue perfusion
For visual assessment of blood vessels, blood flow and related tissue perfusion with OLYMPUS infrared compatible endoscopic imaging system
-
2 DOSAGE AND ADMINISTRATION
2.1 Visual assessment of blood vessels, blood flow and related tissue perfusion
Under sterile conditions, the Indocyanine Green for Injection USP powder should be dissolved with the 10 mL Sterile Water for Injection, USP provided for this product, and the solution used within 6 hours after it is prepared. If a precipitate is present, discard the solution.
The patient should be weighed and the dosage for one administration should be calculated on the basis of 0.1 mg/kg (0.04 mL/kg) - 0.3 mg/kg (0.12 mL/kg) of body weight. Multiple administrations can be performed, up to 2 mg/kg (0.8 mL/kg) per patient.
ICG should be administrated immediately before IR imaging.
Item
Note
Indocyanine Green for Injection, USP
25 mg vials of ICG powder
Sterile Water for Injection (for dissolving ICG)
10 mL vial of sterile water
Syringe (for injecting sterile water into the ICG vial)
Use the syringe whose minimum volume is 10 mL
Syringes (for each administration)
Considering the administration volume, select the appropriate size
Sterile normal saline (for each saline flush)
Approximately 10 – 12 mL following each ICG administration
Syringes (for each saline flush)
Use the syringe whose minimum volume is 12 mL
Prepare the syringes filled with the weight-scaled dose of ICG solution, and the syringes filled with 10 - 12 mL of normal saline for the tight bolus injection.
Immediately before IR imaging, administer the prepared dose of ICG solution intravenously. Immediately follow each ICG injection with a tight bolus injection of approximately 10 - 12 mL of normal saline.
2.2 Visual assessment of the major extrahepatic bile duct
Under sterile conditions, the Indocyanine Green for Injection USP powder should be dissolved with the 10 mL Sterile Water for Injection, USP provided for this product, and the solution used within 6 hours after it is prepared. If a precipitate is present, discard the solution.
The dosage for one administration should be 3.0 mg (1.2 mL) per patient. Multiple administration can be performed, up to 2 mg/kg (0.8 mL/kg) per patient.
ICG should be administrated at least 30 minutes prior to IR imaging.
Item
Note
Indocyanine Green for Injection, USP
25 mg vials of ICG powder
Sterile Water for Injection (for dissolving ICG)
10 mL vial of sterile water
Syringe (for injecting sterile water into the ICG vial)
Use the syringe whose minimum volume is 10 mL
Syringes (for each administration)
Considering the administration volume, select the appropriate size
Sterile normal saline (for each saline flush)
Approximately 10 – 12 mL following each ICG administration
Syringes (for each saline flush)
Use the syringe whose minimum volume is 12 mL
Prepare the syringes filled with 3.0 mg (1.2 mL) of ICG solution, and the syringes filled with 10 - 12 mL of normal saline for the tight bolus.
At least 30 minutes prior to IR imaging, administer the prepared dose of ICG solution intravenously. Immediately follow each ICG injection with a tight bolus injection of approximately 10 - 12 mL of normal saline.
2.3 Viewing intra-operative blood flow in the cerebral area
Under sterile conditions, the Indocyanine Green for Injection USP powder should be dissolved with the 10 mL Sterile Water for Injection, USP provided for this product, and the solution used within 6 hours after it is prepared. If a precipitate is present, discard the solution.
The patient should be weighed and the dosage for one administration should be calculated on the basis of 0.1 mg/kg (0.04 mL/kg) - 0.3 mg/kg (0.12 mL/kg) of body weight. Multiple administration can be performed, up to 2 mg/kg (0.8 mL/kg) per patient.
ICG should be administrated immediately before IR imaging.
Item
Note
Indocyanine Green for Injection, USP
25 mg vials of ICG powder
Sterile Water for Injection (for dissolving ICG)
10 mL vial of sterile water
Syringe (for injecting sterile water into the ICG vial)
Use the syringe whose minimum volume is 10 mL
Syringes (for each administration)
Considering the administration volume, select the appropriate size
Sterile normal saline (for each saline flush)
Approximately 10 – 12 mL following each ICG administration
Syringes (for each saline flush)
Use the syringe whose minimum volume is 12 mL
Prepare the syringes filled with the weight-scaled dose of ICG solution, and the syringes filled with 10 - 12 mL of normal saline for the tight bolus.
Immediately before IR imaging, administer the prepared dose of ICG solution intravenously. Immediately follow each ICG injection with a tight bolus injection of approximately 10 - 12 mL of normal saline.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis
Deaths from anaphylaxis have been reported following Indocyanine Green for Injection USP administration during cardiac catheterization.
5.2 Drug Instability
Indocyanine Green for Injection USP is unstable in aqueous solution and must be used within 6 hours. However, the dye is stable in plasma and whole blood so that samples obtained in discontinuous sampling techniques may be read hours later. Sterile techniques should be used in handling the dye solution as well as in the performance of the dilution curves. If a precipitate is present, discard the solution.
- 6 ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted with Indocyanine Green for Injection USP. It is also not known whether Indocyanine Green for Injection USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Indocyanine Green for Injection USP should be given to a pregnant woman only if clearly indicated.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Indocyanine Green for Injection USP is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of indocyanine green for visual assessment of blood vessels, blood flow and related tissue perfusion, for visual assessment of the major extrahepatic bile duct and for intra-operative blood flow in the cerebral area using this IR imaging application has not been evaluated in pediatric patients.
-
10 OVERDOSAGE
There are no data available describing the signs, symptoms, or laboratory findings accompanying overdosage. The LD50 after intravenous administration ranges between 60 and 80 mg/kg in mice, 50 and 70 mg/kg in rats and 50 and 80 mg/kg in rabbits. Based on body surface area, these doses are 2.4 to 13-fold the maximum recommended human (MRHD) dose of 2 mg/kg for indicator-dilution studies, 10 to 52-fold the MRHD of 0.5 mg/kg for hepatic-function studies, and 7 to 39-fold the MRHD of 0.67 mg/kg for ophthalmic angiography studies.
-
11 DESCRIPTION
Indocyanine Green for Injection USP is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5% sodium iodide. It is packaged with Sterile Water for Injection, USP used to dissolve the indocyanine green. Indocyanine Green for Injection USP is to be administered intravenously.
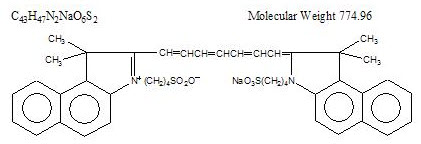
Indocyanine green is a water soluble, tricarbocyanine dye with a peak spectral absorption at 800 nm. The chemical name for Indocyanine Green is 1 H-Benz[e]indolium, 2-[7-[1,3-dihydro-1,1-dimethyl-3-(4-sulfobutyl)-2H-benz[e] indol-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-,hydroxide, inner salt, sodium salt. Indocyanine Green for Injection USP has a pH of approximately 6.5 when reconstituted. Each vial of Indocyanine Green for Injection USP contains 25 mg of indocyanine green as a sterile lyophilized powder.
-
12 CLINICAL PHARMACOLOGY
Indocyanine Green for Injection USP permits recording of the indicator-dilution curves for both diagnostic and research purposes independently of fluctuations in oxygen saturation. Following intravenous injection, Indocyanine Green for Injection USP is rapidly bound to plasma protein, of which albumin is the principle carrier (95%). Indocyanine Green for Injection USP undergoes no significant extrahepatic or enterohepatic circulation; simultaneous arterial and venous blood estimations have shown negligible renal, peripheral, lung or cerebro-spinal uptake of the dye. Indocyanine Green for Injection USP is taken up from the plasma almost exclusively by the hepatic parenchymal cells and is secreted entirely into the bile. After biliary obstruction, the dye appears in the hepatic lymph, independently of the bile, suggesting that the biliary mucosa is sufficiently intact to prevent diffusion of the dye, though allowing diffusion of bilirubin. These characteristics make Indocyanine Green for Injection USP a helpful index of hepatic function.
The peak absorption and emission of Indocyanine Green for Injection USP lie in a region (800 to 850 nm) where transmission of energy by the pigment epithelium is more efficient than in the region of visible light energy. Indocyanine Green for Injection USP also has the property of being nearly 98% bound to blood protein, and therefore, excessive dye extravasation does not take place in the highly fenestrated choroidal vasculature. It is, therefore, useful in both absorption and fluorescence infrared angiography of the choroidal vasculature when using appropriate filters and film in a fundus camera.
The plasma fractional disappearance rate at the recommended 0.5 mg/kg dose has been reported to be significantly greater in women than in men, although there was no significant difference in the calculated value for clearance.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Indocyanine Green for Injection USP is supplied in a kit (NDC: 73624-424-02) containing six 25 mg Indocyanine Green for Injection USP vials and six 10 mL Sterile Water for Injection, USP plastic vials:
NDC: 70100-424-01 Indocyanine Green for Injection USP vial. 25 mg fill in 25 mL vial.
NDC: 63323-185-10 (or NDC: 0409-4887-17) Sterile Water for Injection, USP, 10 mL fill in 10 mL plastic vials.
STORAGE: Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature]. -
Manufactured by:
Patheon Italia S.p.A.
20900 Monza (MB), ITALYDistributed by:
OLYMPUS AMERICA INC.
3500 Corporate Parkway
P.O. Box 610
Center Valley, PA, 18034-0610Sterile Water for Injection,
USP is manufactured by:
Fresenius Kabi USA, LLC
Grand Island, NY 14072 USA
or
Hospira, Inc.
Rocky Mount, NC 27804 USA50652
06/2020 -
PRINCIPAL DISPLAY PANEL - Carton Label
NDC: 73624-424-02
Indocyanine Green
for Injection, USPFor Intravenous Administration 25 mg/Vial Kit Rx Only - Sterile
Distributed by:
OLYMPUS AMERICA INC.Back Panel
NDC: 73624-424-02
DIRECTIONS FOR USE ENCLOSED
CAUTION: To ensure accurate readings,
Indocyanine Green, USP dissolved in Sterile Water
for Injection, USP must be used within 6 hours.
STORAGE: Store at 20° to 25°C (68° to 77°F)
[See USP Controlled Room Temperature].USAGE: See package insert for dosage information.
Contents: Six Indocyanine Green for Injection, USP
vials (25 mg each)
Six Sterile Water for Injection, USP Vials
(10 mL each)Indocyanine Green
for Injection, USPFor Intravenous Administration 25 mg/Vial Kit Rx Only - Sterile
Right Panel
Indocyanine Green
for Injection, USPDistributed by:
OLYMPUS AMERICA INC.
3500 Corporate Parkway, P.O. Box 610,
Center Valley, PA 1834-0610, U.S.A.Manufactured by:
Patheon Italia S.p.A.
20900 Monza (Milano) ITALYSterile Water Manufactured by:
Hospira, Inc.
Rocky Mount, NC 27804
or
Fresenius Kabi USA, LLC
Grand Island, NY 140724/2020
-
PRINCIPAL DISPLAY PANEL - Vial Label
NDC: 70100-424-01
Indocyanine Green
for Injection, USP25 mg/Vial
Single-Patient-Use
After reconstitution, use within 6 hours.Rx only Sterile
Distributed by: Diagnostic Green LLC
50428
04/2022Lot No.
Exp.: -
PRINCIPAL DISPLAY PANEL - STERILE WATER VIAL
10 mL Single-dose
Sterile Water
for Injection, USPFOR DRUG DILUENT USE
LOT ##-###-AA
EXP DMMMYYYYRx only NDC: 0409-4887-17
Contains no antimicrobial or other added
substance. Sterile, nonpyrogenic. Do not give
intravenously unless rendered nearly isotonic.Distributed by RL-7603
Hospira, Inc., Lake Forest, IL 60045 USA -
INGREDIENTS AND APPEARANCE
INDOCYANINE GREEN
indocyanine green and water kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73624-424 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73624-424-02 6 in 1 CARTON 04/01/2021 1 1 in 1 PACKAGE; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1 Part 2 1 VIAL, PLASTIC 10 mL Part 1 of 2 INDOCYANINE GREEN
indocyanine green injection, powder, lyophilized, for solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INDOCYANINE GREEN (UNII: IX6J1063HV) (INDOCYANINE GREEN ACID FORM - UNII:C4V974V932) INDOCYANINE GREEN 25 mg Inactive Ingredients Ingredient Name Strength SODIUM IODIDE (UNII: F5WR8N145C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040811 01/01/2008 Part 2 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 0409-4887 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0409-4887-17 10 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018801 10/27/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040811 04/01/2021 Labeler - Olympus America, Inc. (060321254)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.