equagesic- meprobamate and aspirin tablet
Drug Labeling and Warnings
Drug Details [pdf]
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each tablet of Equagesic, for oral administration, contains 200 mg meprobamate and 325 mg aspirin. Chemically, meprobamate is 2-methyl-2-propyl-1,3- propanediol dicarbamate. Its molecular formula is C9H18N2O4 with a molecular weight of 218.25.
Chemically, aspirin is benzoic acid 2-(acetyloxy)-. Its molecular formula is C9H8O4 with a molecular weight of 180.16. It occurs as an odorless white, needle like crystalline or powdery substance. When exposed to moisture, aspirin hydrolyzes into salicylic and acetic acids, and gives off a vinegary odor. It is highly lipid soluble and slightly soluble in water. The structural formulas of meprobamate and aspirin are:
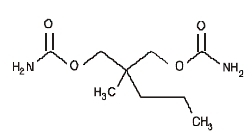
MEPROBAMATE
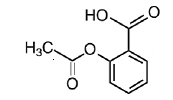
ASPIRIN
The inactive ingredients present are D&C Yellow 10, FD&C Red 3, FD&C Yellow 6, hydrogenated vegetable oil, magnesium stearate, microcrystalline cellulose, polacrilin potassium, and starch.
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
As an adjunct in the short-term treatment of pain accompanied by tension and/or anxiety in patients with musculoskeletal disease. Clinical trials have demonstrated that in these situations relief of pain is somewhat greater than with aspirin alone. Equagesic is not intended for use longer than 10 days.
-
Contraindications
Usage in Pregnancy and Lactation
An increased risk of congenital malformations associated with the use of minor tranquilizers (meprobamate, chlordiazepoxide, and diazepam) during the first trimester of pregnancy has been suggested in several studies. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided.
Because of the known effect of non-steroidal anti-inflammatory drugs (NSAIDs) on the fetal cardiovascular system (closure of the ductus arteriosus), use during the third trimester of pregnancy should be avoided. Salicylate products have also been associated with alterations in maternal and neonatal hemostasis mechanisms, decreased birth weight, and perinatal mortality. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physicians about the desirability of discontinuing the drug. Meprobamate passes the placental barrier. It is present both in umbilical-cord blood at or near maternal plasma levels and in breast milk of lactating mothers at concentrations two to four times that of maternal plasma. When use of meprobamate is contemplated in breast-feeding patients, the drug’s higher concentrations in breast milk as compared to maternal plasma levels should be considered.
Equagesic is contraindicated in patients with acute intermittent porphyria and in patients with allergic or idiosyncratic reactions to aspirin, meprobamate, or related compounds, such as carbromal, carisoprodol, mebutamate, nonsteroidal anti-inflammatory drug products, salicylates, or tybamate. Equagesic is also contraindicated in patients with the syndrome of asthma, rhinitis, and nasal polyps. The aspirin component of Equagesic may cause severe angioedema, bronchospasm (asthma), or urticaria. Reye’s syndrome: Aspirin should not be used in children or teenagers for viral infections, with or without fever, because of the risk of Reye’s syndrome with concomitant use of aspirin in certain viral illnesses.
-
WARNINGS
Equagesic should be prescribed cautiously and in small quantities to patients with suicidal tendencies.
Additive Effects: Since CNS-suppressant effects of meprobamate and alcohol or meprobamate and other psychotropic drugs may be additive, appropriate caution should be exercised with patients who take more than one of these agents simultaneously.
Alcohol Warning: Patients who consume three or more alcoholic drinks every day should be counseled about the bleeding risks involved with chronic, heavy alcohol use while taking aspirin.
Coagulation Abnormalities: Even low doses of aspirin can inhibit platelet function leading to an increase in bleeding time. This can adversely affect patients with inherited (hemophilia) or acquired (liver disease or vitamin K deficiency) bleeding disorders.
Gastrointestinal Side Effects (GI): GI side effects include gross GI bleeding, heartburn, nausea, stomach pain, and vomiting. Although minor upper GI symptoms, such as dyspepsia, are common and can occur anytime during therapy, physicians should remain alert for signs of ulceration and bleeding, even in the absence of previous GI symptoms. Physicians should inform patients about the signs and symptoms of GI side effects and what steps to take if they occur.
Peptic Ulcer Disease: Patients with a history of active peptic ulcer disease should avoid using aspirin, which can cause gastric mucosal irritation and bleeding.
Potentially Hazardous Tasks
Patients should be warned that meprobamate may impair the mental and/or physical abilities required for performance of potentially hazardous tasks, such as driving a motor vehicle or operating machinery. Such tasks should be avoided while taking this product.
-
PRECAUTIONS
General
Equagesic should be prescribed with caution in certain special-risk populations, such as elderly or debilitated patients and those with acute abdominal conditions, Addison’s disease, coagulation disorders, elevated intracranial pressure, head injuries, hypothyroidism, impairment of liver or kidney function, prostatic hypertrophy, or urethral stricture. Meprobamate is metabolized in the liver and excreted by the kidney. To avoid its excess accumulation, caution should be exercised in the administration to patients with compromised liver or kidney function. Meprobamate occasionally may precipitate seizures in epileptic patients.
Information for Patients
Patients should be informed that Equagesic contains aspirin and should not be taken by patients with an aspirin allergy.
Patients with a predisposition for gastrointestinal bleeding should be cautioned that concomitant use of medications containing aspirin and/or alcohol may have an additive effect in this regard.
Drug Interactions
Angiotensin Converting Enzyme (ACE) Inhibitors: The hyponatremic and hypotensive effects of ACE inhibitors may be diminished by the concomitant administration of aspirin due to its indirect effect on the reninangiotensin conversion pathway.
Acetazolamide: Concurrent use of aspirin and acetazolamide can lead to high serum concentrations of acetazolamide (and toxicity) due to competition at the renal tubule for secretion.
Alcohol, General Anesthetics, Narcotic Analgesics, Sedative Hypnotics, Tranquilizers such as Chlordiazepoxide, or Other CNS Depressants: The effects of these substances may be enhanced, causing increased CNS depression.
Anticoagulant Therapy (Heparin and Warfarin): Patients on anticoagulation therapy are at increased risk for bleeding because of drug-drug interactions and the effect on platelets. Aspirin can displace warfarin from protein binding sites, leading to prolongation of both the prothrombin time and the bleeding time. Aspirin can increase the anticoagulant activity of heparin, increasing bleeding risk.
Anticonvulsants: Salicylates can displace protein-bound phenytoin and valproic acid, leading to a decrease in the total concentration of phenytoin and an increase in serum valproic acid levels.
Beta Blockers: The hypotensive effects of beta blockers may be diminished by the concomitant administration of aspirin due to inhibition of renal prostaglandins, leading to decreased renal blood flow and salt and fluid retention.
Corticosteroids: In patients receiving concomitant corticosteroids and chronic use of medications containing aspirin, withdrawal of corticosteroids may result in salicylism because corticosteroids enhance renal clearance of salicylates and their withdrawal is followed by return to normal rates of renal clearance.
Diuretics: The effectiveness of diuretics in patients with underlying renal or cardiovascular disease may be diminished by the concomitant administration of aspirin due to inhibition of renal prostaglandins, leading to decreased renal blood flow and salt and fluid retention.
6-Mercaptopurine and Methotrexate: Bone marrow toxicity and blood dyscrasia may result from displacing these drugs from secondary binding sites, and in the case of methotrexate, also reducing its excretion.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): The concurrent use of aspirin with other NSAIDs should be avoided because this may increase bleeding or lead to decreased renal function.
Oral Hypoglycemics: Moderate doses of aspirin may increase the effectiveness of oral hypoglycemic drugs, leading to hypoglycemia.
Uricosuric Agents (Probenicid and Sulfinpyrazone): Salicylates antagonize the uricosuric action, reducing their effectiveness in the treatment of gout. Aspirin competes with these agents for protein binding sites.
Laboratory Test Interactions
Aspirin may interfere with the following laboratory determinations in blood: blood urea nitrogen, cholesterol, elevated hepatic enzymes including aspartate aminotransferase (AST), fasting blood glucose, hyperkalemia, prolonged bleeding time, protein, prothrombin time, serum amylase, serum creatinine, and uric acid. Aspirin may interfere with the following laboratory determinations in urine: 5-hydroxyindoleacetic acid, diacetic acid, Gerhardt ketone, glucose, proteinuria, uric acid, spectrophotometric detection of barbiturates, and vanillylmandelic acid (VMA).
Carcinogenesis, Mutagenesis Impairment of Fertility
Administration of aspirin for 68 weeks at 0.5 percent in the feed of rats was not carcinogenic. In the Ames Salmonella assay, aspirin was not mutagenic; however, aspirin did induce chromosome aberrations in cultured human fibroblasts.
Labor and Delivery
Aspirin should be avoided during the third trimester of pregnancy and during labor and delivery because it can result in excessive blood loss at delivery. Prolonged gestation and prolonged labor due to prostaglandin inhibition have been reported.
Nursing Mothers
Nursing mothers should avoid using aspirin because salicylate is excreted in breast milk. Use of high doses may lead to rashes, platelet abnormalities, and bleeding in nursing infants. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother ( See also Contraindications).
Pediatric Use
Safety and effectiveness have not been established for pediatric patients under the age of 12 years (See Contraindications).
Geriatric Use
Clinical studies of meprobamate with aspirin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
Adverse Reactions
Body as a Whole
Fever, hypothermia, thirst.
Allergic or Idiosyncratic
Severe hypersensitivity reactions, including anaphylaxis, angioneurotic edema, anuria, asthma, bronchospasm, bullous dermatitis, chills, erythema multiforme, exfoliative erythroderma, laryngeal edema, oliguria, proctitis, purpura, Stevens-Johnson syndrome, stomatitis, and urticaria. Milder reactions are characterized by an itchy, erythematous maculopapular, or urticarial rash which may be generalized or confined to the groin. Other reactions have included acute nonthrombocytopenic purpura, adenopathy, cross-sensitivity between meprobamate/ mebutamate and meprobamate/carbromal, ecchymoses, eosinophilia, fixed-drug eruption with cross-reaction to carisoprodol, leukopenia, peripheral edema, and petechiae.
Cardiovascular
Various forms of arrhythmia, hypotension, palpitation, syncope, tachycardia, and transient ECG changes.
Central Nervous System
Agitation, ataxia, cerebral edema, coma, confusion, dizziness, drowsiness, dysphoria, euphoria, fast EEG activity, headache, impairment of visual accommodation, lethargy, overstimulation, paradoxical excitement, paresthesias, sedation, slurred speech, subdural or intracranial hemorrhage, seizures, vertigo, and weakness.
Fluid and Electrolyte
Dehydration, hyperkalemia, metabolic acidosis, and respiratory alkalosis.
Gastrointestinal
Abdominal pain, constipation, diarrhea, dyspepsia, epigastric discomfort, gastric distress, gastrointestinal bleeding, heartburn, hepatitis, nausea, pancreatitis, Reye’s syndrome, transient elevations of hepatic enzymes, ulceration and perforation, and vomiting.
Hematologic (see also “Allergic or Idiosyncratic”)
Agranulocytosis and aplastic anemia have been reported, although no causal relationship has been established, coagulopathy, disseminated intravascular coagulation, exacerbation of porphyric symptoms, hemolytic anemia, iron deficiency anemia, occult blood loss, prolongation of the prothrombin time, thrombocytopenia, and thrombocytopenic purpura.
Musculoskeletal
Rhabdomyolysis
Metabolism
Hyperglycemia and hypoglycemia
Reproductive
Prolonged pregnancy and labor, stillbirths, lower birth weight infants, and antepartum and postpartum bleeding.
Respiratory
Acute airway obstruction, hyperpnea, pulmonary edema, and tachypnea.
Special Senses
Hearing loss and tinnitus.
Urogenital
Interstitial nephritis, papillary necrosis, proteinuria, and renal insufficiency and failure.
-
Drug Abuse and Dependence
Physical dependence, psychological dependence, and abuse have occurred. Chronic intoxication from prolonged ingestion of, usually, greater-than-recommended doses is manifested by ataxia, slurred speech, and vertigo. Therefore, careful supervision of dose and amounts prescribed is advised, as well as avoidance of prolonged administration, especially for alcoholics and other patients with a known propensity for taking excessive quantities of drugs. Sudden withdrawal of the drug after prolonged and excessive use may precipitate recurrence of preexisting symptoms, such as anorexia, anxiety, or insomnia, or withdrawal reactions, such as ataxia, confusional states, hallucinosis, muscle twitching, tremors, vomiting, and, rarely, convulsive seizures. Such seizures are more likely to occur in persons with central nervous system damage or preexistent or latent convulsive disorders. Onset of withdrawal symptoms occurs usually within 12 to 48 hours after discontinuation of meprobamate; symptoms usually cease within the next 12- to 48-hour period. When excessive dosage has continued for weeks or months, dosage should be reduced gradually over a period of 1 to 2 weeks rather than abruptly stopped. Alternatively, a long-acting barbiturate may be substituted, then gradually withdrawn.
-
Overdosage
Treatment of overdose with Equagesic is essentially symptomatic and supportive. In cases where excessive doses of Equagesic have been taken, sleep ensues rapidly and blood pressure, pulse, and respiratory rates are reduced to basal levels. Any drug remaining in the stomach should be removed and symptomatic treatment given. After emesis and/or lavage, activated charcoal may reduce absorption of both aspirin and meprobamate. Should respiration or blood pressure become compromised, respiratory assistance, central nervous system stimulants, and pressor agents should be administered cautiously as indicated. Diuresis, osmotic (mannitol) diuresis, peritoneal dialysis, and hemodialysis have been used successfully in removing both aspirin and meprobamate. Alkalinization of the urine increases the excretion of salicylates. Careful monitoring of urinary output is necessary, and caution should be taken to avoid overhydration. Relapse and death, after initial recovery, have been attributed to incomplete gastric emptying and delayed absorption. Salicylate toxicity may result from acute ingestion (overdose) or chronic intoxication. Signs and symptoms include abdominal pain, acid-base disturbances with development of metabolic acidosis, convulsions, delirium, hyperpnea, hyperthermia, hypoprothrombinemia, restlessness, Tinnitus (ringing in the ears), and vomiting. The early signs of salicylic overdose (salicylism), including tinnitus, occur at plasma concentrations approaching 200 μg/mL. Plasma concentrations of aspirin above 300 μg/mL are clearly toxic. Severe toxic effects are associated with levels above 400 μg/mL. A single lethal dose of aspirin in adults is not known with certainty but death may be expected at 30 g. For real or suspected overdose, a Poison Control Center should be contacted immediately. Careful medical management is essential. In acute aspirin overdose, severe acid-base and electrolyte disturbances may occur and are complicated by hyperthermia and dehydration. Respiratory alkalosis occurs early while hyperventilation is present, but is quickly followed by metabolic acidosis. Treatment of aspirin overdose consists primarily of supporting vital functions, increasing salicylate elimination, and correcting the acid-base disturbance. Gastric emptying and/or lavage is recommended as soon as possible after ingestion, even if the patient has vomited spontaneously. After lavage and/or emesis administration of activated charcoal, as a slurry, is beneficial, if less than 3 hours have passed since ingestion. Charcoal adsorption should not be employed prior to emesis and lavage.
Severity of aspirin intoxication is determined by measuring the blood salicylate level. Acid-base status should be closely followed with serial blood gas and serum pH measurements. Fluid and electrolyte balance should also be maintained. In severe cases, hyperthermia and hypovolemia are the immediate threats to life. Children should be sponged with tepid water. Replacement fluid should be administered intravenously and augmented with correction of acidosis. Plasma electrolytes and pH should be monitored to promote alkaline diuresis of salicylate if renal function is normal. Infusion of glucose may be required to control hypoglycemia.
Hemodialysis and peritoneal dialysis can be performed to reduce the body drug content. In patients with renal insufficiency or in cases of life-threatening intoxication, dialysis is usually required. Exchange transfusion may be indicated in infants and young children. Suicidal attempts with meprobamate have resulted in ataxia, coma, drowsiness, lethargy, shock, stupor, and respiratory and vasomotor collapse. Some suicidal attempts have been fatal. The following data have been reported in the literature and from other sources. These data are not expected to correlate with each case (considering factors such as individual susceptibility and length of time from ingestion to treatment) but represent the usual ranges reported. Acute simple overdose (meprobamate alone): Death has been reported with ingestion of as little as 12 grams meprobamate and survival with as much as 40 grams.
BLOOD LEVELS
0.5 to 2 mg percent represents the usual blood-level range of meprobamate after therapeutic doses. 3 to 10 mg percent usually corresponds to findings of mild to moderate symptoms of overdosage, such as stupor or light coma. 10 to 20 mg percent usually corresponds to deeper coma, requiring more intensive treatment. Some fatalities occur. At levels greater than 20 mg percent, more fatalities than survivals can be expected. Acute combined overdose (meprobamate with other CNS psychotropic drugs or alcohol): Since effects can be additive, a history of ingestion of a low dose of meprobamate plus any of these compounds (or of a relatively low blood or tissue level) cannot be used as a prognostic indicator.
- Dosage and Administration
-
How Supplied
Equagesic® (meprobamate and aspirin tablets) Tablets, 200 mg meprobamate and 325 mg aspirin, are available as follows: NDC: 10551-091-10, pink and yellow, double-layer, round, scored tablet marked “LP” and “91”, in bottles of 100 tablets.
Store at controlled room temperature, 20°-25°C (68°-77°F).
Protect from moisture.
Keep tightly closed.
Protect from light.
Dispense in light-resistant, tight container.
Manufactured for:
Leitner Pharmaceuticals™, LLC
Bristol, TN 37620
www.leitnerpharma.com
Manufactured by:
Mikart, Inc.
Atlanta, GA 30318
1019A00
09/06
1-0047-1
-
INGREDIENTS AND APPEARANCE
EQUAGESIC
meprobamate and aspirin tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10551-091 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength meprobamate (UNII: 9I7LNY769Q) (meprobamate - UNII:9I7LNY769Q) 200 mg aspirin (UNII: R16CO5Y76E) (aspirin - UNII:R16CO5Y76E) 325 mg Inactive Ingredients Ingredient Name Strength D&C Yellow 10 () FD&C Red 3 () FD&C Yellow 6 () hydrogenated vegetable oil () magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose () polacrilin potassium () starch () Product Characteristics Color YELLOW, PINK Score 2 pieces Shape ROUND Size 11mm Flavor Imprint Code LP;91 Contains Coating false Symbol false Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10551-091-10 100 in 1 BOTTLE, PLASTIC Labeler - Mikart, Inc.
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.