nandrolone decanoate- Nandrolone Decanoate injection
Drug Labeling and Warnings
Drug Details [pdf]
- N/A - Section Title Not Found In Database
-
DESCRIPTION
A sterile oleaginous solution containing per mL: Nandrolone Decanoate 100 mg with Benzyl Alcohol 10% as solubilizer/preservative, in Sesame Oil q.s., and Nandrolone Decanoate 200 mg with Benzyl Alcohol 5% as solubilizer/preservative, in Sesame Oil q.s.
Nandrolone decanoate (C28H44O3) occurs as a fine, white to creamy white, crystalline powder. It is odorless, or may have a slight odor.
Nandrolone decanoate is soluble in chloroform, in alcohol, in acetone, and in vegetable oils. It is practically insoluble in water.
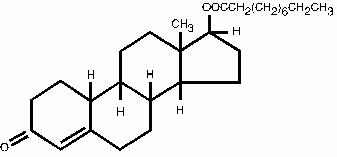
-
CLINICAL PHARMACOLOGY
Anabolic steroids are synthetic derivatives of testosterone. Certain clinical effects and adverse reactions demonstrate the androgenic properties of this class of drugs. Complete dissociation of anabolic and androgenic effects has not been achieved. The actions of anabolic steroids are therefore similar to those of male sex hormones with the possibility of causing serious disturbances of growth and sexual development if given to young children. Anabolic steroids suppress the gonadotropic functions of the pituitary and may exert a direct effect upon the testis.
Anabolic steroids have been reported to increase low-density lipoproteins and decrease high-density lipoproteins. These changes revert to normal on discontinuation of treatment.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
-
Male patients with carcinoma of the breast or with known or suspected carcinoma of the prostate.
-
Carcinoma of the breast in females with hypercalcemia: androgenic anabolic steroids may stimulate osteolytic resorption of bones.
-
Pregnancy, because of masculinization of the fetus.
-
Nephrosis or the nephrotic phase of nephritis.
-
-
WARNINGS
PELIOSIS HEPATIS, A CONDITION IN WHICH LIVER AND SOMETIMES SPLENIC TISSUE IS REPLACED WITH BLOOD-FILLED CYSTS, HAS BEEN REPORTED IN PATIENTS RECEIVING ANDROGENIC ANABOLIC STEROID THERAPY. THESE CYSTS ARE SOMETIMES PRESENT WITH MINIMAL HEPATIC DYSFUNCTION, BUT AT OTHER TIMES THEY HAVE BEEN ASSOCIATED WITH LIVER FAILURE. THEY ARE OFTEN NOT RECOGNIZED UNTIL LIFE-THREATENING LIVER FAILURE OR INTRA-ABDOMINAL HEMORRHAGE DEVELOPS. WITHDRAWAL OF DRUG USUALLY RESULTS IN COMPLETE DISAPPEARANCE OF LESIONS. LIVER CELL TUMORS ARE ALSO REPORTED. MOST OFTEN THESE TUMORS ARE BENIGN AND ANDROGEN-DEPENDENT, BUT FATAL MALIGNANT TUMORS HAVE BEEN REPORTED. WITHDRAWAL OF DRUG OFTEN RESULTS IN REGRESSION OR CESSATION OF PROGRESSION OF THE TUMOR. HOWEVER, HEPATIC TUMORS ASSOCIATED WITH ANDROGENS OR ANABOLIC STEROIDS ARE MUCH MORE VASCULAR THAN OTHER HEPATIC TUMORS AND MAY BE SILENT UNTIL LIFE-THREATENING INTRA-ABDOMINAL HEMORRHAGE DEVELOPS. BLOOD LIPID CHANGES THAT ARE KNOWN TO BE ASSOCIATED WITH INCREASED RISK OF ATHEROSCLEROSIS ARE SEEN IN PATIENTS TREATED WITH ANDROGENS AND ANABOLIC STEROIDS. THESE CHANGES INCLUDE DECREASED HIGH-DENSITY LIPOPROTEIN AND SOMETIMES INCREASED LOW-DENSITY LIPOPROTEIN. THE CHANGES MAY BE VERY MARKED AND COULD HAVE A SERIOUS IMPACT ON THE RISK OF ATHEROSCLEROSIS AND CORONARY ARTERY DISEASE. Hypercalcemia may develop both spontaneously and as a result of androgen therapy in women with disseminated breast carcinoma. If it develops while on this agent, the drug should be discontinued. Caution is required in administering these agents to patients with cardiac, renal or hepatic disease. Cholestatic jaundice is associated with therapeutic use of anabolic and androgenic steroids. Edema may occur occasionally with or without congestive heart failure. Concomitant administration of adrenal steroids or ACTH may add to the edema.
In children, anabolic steroid treatment may accelerate bone maturation without producing compensatory gain in linear growth. This adverse effect may result in compromised adult stature. The younger the child the greater the risk of compromising final mature height. The effect on bone maturation should be monitored by assessing bone age of the wrist and hand every six months.
This drug has not been shown to be safe and effective for the enhancement of athletic performance. Because of the potential risk of serious adverse health effects, this drug should not be used for such purpose.
-
PRECAUTIONS
General
Women should be observed for signs of virilization (deepening of the voice, hirsutism, acne, clitorimegaly and menstrual irregularities). Discontinuation of drug therapy at the time of evidence of mild virilism is necessary to prevent irreversible virilization. Such virilization is usual following anabolic steroid use in high doses.
The insulin or oral hypoglycemic dosage may need adjustment in diabetic patients who receive anabolic steroids.
Information for patients
The physician should instruct patients to report any of the following side effects of androgenic anabolic steroids:
Hoarseness, acne, changes in menstrual periods, more hair on the face, nausea, vomiting, changes in skin color, or ankle swelling.
Laboratory tests
Women with disseminated breast carcinoma should have frequent determination of urine and serum calcium levels during the course of anabolic therapy (see WARNINGS section).
If children are treated, periodic (every six months) X-ray examinations of bone age should be made during treatment to determine the rate of bone maturation and the effects of anabolic therapy on the epiphyseal centers.
Hemoglobin and hematocrit should be checked periodically for polycythemia in patients who are receiving high doses of anabolic steroids.
Serum lipids and high-density lipoprotein cholesterol should be determined periodically.
Because of the hepatotoxicity associated with the use of 17-alpha-alkylated anabolic steroids, liver function tests should be obtained periodically.
Drug interactions
Anticoagulants. Anabolic steroids may increase sensitivity to oral anticoagulants. Dosage of the anticoagulant may have to be decreased in order to maintain the prothrombin time at the desired therapeutic level. Patients receiving oral anticoagulant therapy require close monitoring, especially when anabolic steroids are started or stopped.
Drug/laboratory test interactions
Anabolic steroid therapy may decrease thyroxine-binding globulin resulting in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged.
Anabolic steroids may cause an increase in prothrombin time.
Carcinogenesis, mutagenesis, impairment of fertility
Nandrolone decanoate has not been tested in laboratory animals for carcinogenic or mutagenic effects. Liver cell tumors have been reported in patients receiving androgenic anabolic steroid therapy (see WARNINGS section). Geriatric patients treated with anabolics may be at an increased risk for prostatic hypertrophy and prostatic carcinoma.
Nursing mothers
It is not known whether anabolic steroids are excreted in human milk. Many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from anabolic steroids, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric use
The safety and efficacy of nandrolone decanoate in children with metastatic breast cancer (rarely found) has not been established. Anabolic agents may accelerate epiphyseal maturation more rapidly than linear growth in children, and the effect may continue for six months after the drug has been stopped. Therefore, therapy should be monitored by X-ray studies at six month intervals in order to avoid the risk of compromising the adult height.
-
ADVERSE REACTIONS
Hepatic:
Hepatocellular neoplasms and peliosis hepatis have been reported in association with long-term androgenic anabolic steroid therapy (see WARNINGS section).
Genitourinary System:
In men. a. Prepubertal: Phallic enlargement and increased frequency of erections. b. Postpubertal: Inhibition of testicular function, testicular atrophy and oligospermia, impotence, chronic priapism, epididymitis and bladder irritability.
In women: Clitoral enlargement, menstrual irregularities.
In both sexes: Increased or decreased libido.
CNS:
Habituation, excitation, insomnia, depression.
Gastrointestinal:
Nausea, vomiting, diarrhea.
Hematologic:
Bleeding in patients on concomitant anticoagulant therapy (see PRECAUTIONS, Drug Interactions).
Breast:
Gynecomastia.
Larynx:
Deepening of the voice in women.
Hair:
Hirsutism and male pattern of baldness in women.
Skin:
Acne (especially in women and prepubertal boys.)
Skeletal:
Premature closure of epiphyses in children (see PRECAUTIONS, Pediatric use).
Fluid and Electrolytes:
Edema, retention of serum electrolytes (sodium, chloride, potassium, phosphate, calcium).
Metabolic/Endocrine:
Decreased glucose tolerance (see PRECAUTIONS, General), increased serum levels of low-density lipoprotein and decreased levels of high-density lipoprotein (see PRECAUTIONS, Laboratory tests), increased creatine and creatinine excretion, increased serum levels of creatinine phosphokinase (CPK). Some virilizing changes in women are irreversible even after prompt discontinuance of therapy and are not prevented by concomitant use of estrogens (see PRECAUTIONS).
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Nandrolone decanoate injection is intended for deep intramuscular injection only, into the gluteal muscle preferably.
Dosage should be based on therapeutic response and consideration of the benefit to risk ratio. Duration of therapy will depend on the response of the condition and the appearance of adverse reactions. If possible, therapy should be intermittent.
Nandrolone decanoate should be regarded as adjunctive therapy and adequate quantities of nutrients should be consumed in order to obtain maximal therapeutic effects. For example, when it is used in the treatment of refractory anemia, adequate iron intake is required for a maximal response.
Anemia of Renal Disease
A dose of 50 to 100 mg per week is recommended for women and 100 to 200 mg per week for men. Drug therapy should be discontinued if no hematologic improvement is seen within the first six months. For children from 2 to 13 years of age, the average dose is 25 to 50 mg every 3 to 4 weeks.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
-
HOW SUPPLIED
Nandrolone Decanoate Injection USP, 100 mg per mL is available in multiple dose vials of 2 mL, in cartons of 10.
Nandrolone Decanoate Injection USP, 200 mg per mL is available in single dose vials of 1 mL, in cartons of 25.
Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature].
PROTECT FROM LIGHT. Store in carton until contents are used.
Literature revised: March 2006Product Nos.: 0432-02, 0710-01
Watson Laboratories, Inc.
Corona, CA 92880 USA
-
INGREDIENTS AND APPEARANCE
NANDROLONE DECANOATE
nandrolone decanoate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0591-6717 Route of Administration INTRAMUSCULAR DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nandrolone Decanoate (UNII: H45187T098) (Nandrolone - UNII:6PG9VR430D) 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzyl Alcohol (UNII: LKG8494WBH) Sesame Oil (UNII: QX10HYY4QV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0591-6717-47 10 in 1 CARTON 1 NDC: 0591-6717-02 2 mL in 1 VIAL, MULTI-DOSE NANDROLONE DECANOATE
nandrolone decanoate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0591-2186 Route of Administration INTRAMUSCULAR DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nandrolone Decanoate (UNII: H45187T098) (Nandrolone - UNII:6PG9VR430D) 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzyl Alcohol (UNII: LKG8494WBH) Sesame Oil (UNII: QX10HYY4QV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0591-2186-53 25 in 1 CARTON 1 NDC: 0591-2186-54 1 mL in 1 VIAL, SINGLE-DOSE Labeler - Watson Laboratories, Inc.
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.