INBRIJA- levodopa capsule
Inbrija by
Drug Labeling and Warnings
Inbrija by is a Prescription medication manufactured, distributed, or labeled by Acorda Therapeutics, Inc., Avista Pharma Solutions, Inc., Boston Analytical, Inc., Catalent Massachusetts LLC, Sharp Corporation, Ajinomoto Company, Inc., PPD Development Ireland Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use INBRIJA safely and effectively. See full prescribing information for INBRIJA.
INBRIJA™ (levodopa inhalation powder), for oral inhalation use
Initial U.S. Approval: 1970INDICATIONS AND USAGE
INBRIJA is an aromatic amino acid indicated for the intermittent treatment of OFF episodes in patients with Parkinson's disease treated with carbidopa/levodopa ( 1)
DOSAGE AND ADMINISTRATION
- For oral inhalation only. DO NOT swallow INBRIJA capsules. Only use INBRIJA capsules with the INBRIJA inhaler ( 2.1)
- Inhale the contents of two INBRIJA capsules (84 mg) as needed for OFF symptoms, up to 5 times daily ( 2.2)
- The maximum dose per OFF period is 84 mg, and the maximum recommended daily dosage of INBRIJA is 420 mg ( 2.2)
DOSAGE FORMS AND STRENGTHS
Inhalation powder: INBRIJA capsules contain 42 mg levodopa for use with the INBRIJA inhaler ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- May cause falling asleep during activities of daily living ( 5.1)
- Avoid sudden discontinuation or rapid dose reduction to reduce the risk of withdrawal-emergent hyperpyrexia and confusion ( 5.2)
- Hallucinations/exacerbation of psychosis may occur. Patients with a major psychotic disorder should not be treated with INBRIJA ( 5.3, 7.2)
- Impulse Control Disorders: consider dose reduction or stopping INBRIJA ( 5.4)
- May cause or exacerbate dyskinesia: adjustment of levodopa therapy may be considered, including stopping INBRIJA ( 5.5)
- Not recommended in patients with asthma, COPD, or other chronic underlying lung disease ( 5.6)
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 5% and higher than placebo) were cough, nausea, upper respiratory tract infection, and sputum discolored ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Acorda Therapeutics, Inc. at 1-800-367-5109 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Falling Asleep During Activities of Daily Living and Somnolence
5.2 Withdrawal-Emergent Hyperpyrexia and Confusion
5.3 Hallucinations/Psychosis
5.4 Impulse Control/Compulsive Behaviors
5.5 Dyskinesia
5.6 Bronchospasm in Patients with Lung Disease
5.7 Glaucoma
5.8 Laboratory Test Abnormalities
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase (MAO) Inhibitors
7.2 Dopamine D2 Receptor Antagonists and Isoniazid
7.3 Iron Salts
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
INBRIJA capsules are for oral inhalation only and should be used only with the INBRIJA inhaler.
2.1 Important Administration Instructions
INBRIJA capsules are for oral inhalation only and should be used only with the INBRIJA inhaler. INBRIJA capsules must not be swallowed as the intended effect will not be obtained. INBRIJA capsules should be stored in their blister package and only removed immediately before use [see How Supplied/Storage and Handling (16.2)] .
2.2 Recommended Dosage
INBRIJA should be taken when symptoms of an OFF period start to return.
The recommended dosage of INBRIJA is oral inhalation of the contents of two 42 mg capsules (84 mg) as needed, up to 5 times a day. The maximum dose per OFF period is 84 mg, and the maximum daily dosage is 420 mg. INBRIJA has been shown to be effective only in combination with carbidopa/levodopa [see Indications and Usage (1)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
INBRIJA is contraindicated in patients currently taking a nonselective monoamine oxidase (MAO) inhibitor (e.g., phenelzine and tranylcypromine) or who have recently (within 2 weeks) taken a nonselective MAO inhibitor. Hypertension can occur if these drugs are used concurrently [see Drug Interactions (7.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Falling Asleep During Activities of Daily Living and Somnolence
Patients treated with levodopa, the active ingredient in INBRIJA, have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes resulted in accidents. Although many of these patients reported somnolence, some reported no warning signs (sleep attack) and believed that they were alert immediately prior to the event. Some of these events have been reported more than 1 year after the initiation of treatment.
Prescribers should reassess patients for drowsiness or sleepiness. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
Before initiating treatment with INBRIJA, advise patients about the potential to develop drowsiness and ask about factors that may increase the risk for somnolence with INBRIJA such as the concomitant use of sedating medications and the presence of sleep disorders. Consider discontinuing INBRIJA in patients who report significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.).
If treatment with INBRIJA continues, patients should be advised not to drive and to avoid other activities that might result in harm if the patients become somnolent. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
5.2 Withdrawal-Emergent Hyperpyrexia and Confusion
A symptom complex that resembles neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in dopaminergic therapy.
5.3 Hallucinations/Psychosis
In placebo-controlled trials [see Clinical Studies (14)], hallucinations were reported in less than 2% of patients treated with INBRIJA. Hallucinations may be responsive to reducing levodopa therapy. Hallucinations may be accompanied by confusion, insomnia, and excessive dreaming. Abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
Because of the risk of exacerbating psychosis, patients with a major psychotic disorder should ordinarily not be treated with INBRIJA. In addition, medications that antagonize the effects of dopamine used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of INBRIJA [see Drug Interactions (7.2)].
5.4 Impulse Control/Compulsive Behaviors
Patients treated with INBRIJA can experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued.
Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with INBRIJA. Consider stopping the medication if a patient develops such urges while taking INBRIJA.
5.5 Dyskinesia
INBRIJA may cause or exacerbate dyskinesias. If troublesome dyskinesias occur, prescribers may need to consider stopping treatment with INBRIJA and/or adjusting the patient's daily medications for the treatment of Parkinson's disease. In Study 1, 4% of patients treated with INBRIJA 84 mg reported dyskinesia, compared with 1% for patients on placebo [see Adverse Reactions (6.1)] .
5.6 Bronchospasm in Patients with Lung Disease
Because of the risk of bronchospasm, use of INBRIJA in patients with asthma, COPD, or other chronic underlying lung disease is not recommended.
In a double-blind, placebo-controlled, crossover clinical study, 25 otherwise healthy subjects with mild or moderate asthma on a stable regimen of asthma medication received placebo or INBRIJA 84 mg every 4 hours for a total of three doses. Cough was the most frequent adverse reaction, reported by 60% of subjects following administration of INBRIJA and 0% following administration of placebo. Following administration of INBRIJA, 10 subjects (40%) had temporary reductions from baseline (between 15% and 59%) for FEV 1; 4 of these subjects also had a reduction in FEV 1 following administration of placebo. Subjects with a reduction in FEV 1 remained asymptomatic and did not require rescue treatment.
5.7 Glaucoma
INBRIJA may cause increased intraocular pressure in patients with glaucoma. Monitor patients for increased intraocular pressure during therapy with INBRIJA.
5.8 Laboratory Test Abnormalities
Abnormalities in laboratory tests may include elevations of liver function tests such as alkaline phosphatase, AST, ALT, lactic dehydrogenase (LDH), and bilirubin. Abnormalities in blood urea nitrogen (BUN), hemolytic anemia and positive direct antibody test have also been reported.
Patients taking levodopa or carbidopa-levodopa may have increased levels of catecholamines and their metabolites in plasma and urine giving false positive results suggesting the diagnosis of pheochromocytoma in patients on levodopa and carbidopa-levodopa.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in the labeling:
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.1)]
- Withdrawal-Emergent Hyperpyrexia and Confusion [see Warnings and Precautions (5.2)]
- Hallucinations/Psychosis [see Warnings and Precautions (5.3)]
- Impulse Control/Compulsive Behaviors [see Warnings and Precautions (5.4)]
- Dyskinesia [see Warnings and Precautions (5.5)]
- Bronchospasm in Patients with Lung Disease [see Warnings and Precautions (5.6)]
- Glaucoma [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions in Study 1
Table 1 lists the adverse reactions that occurred in at least 2% of patients with Parkinson's disease who were treated with INBRIJA 84 mg and higher than placebo for OFF periods in Study 1 [see Clinical Studies (14)]. Study 1 was a double-blind, placebo-controlled study, in which 114 patients received INBRIJA 84 mg (two 42 mg capsules) for an average of 2 doses per day, to a maximum of 5 times a day, and 112 patients received placebo. INBRIJA-treated patients were 45-82 years of age (mean 63.5 years of age) and were predominantly male (72%) and white (94%). All patients were also treated with oral carbidopa/levodopa. The most common adverse reactions (≥ 5% and higher than placebo) in Study 1 were cough, nausea, upper respiratory tract infection, and sputum discolored.
Table 1: Adverse Reactions at an Incidence ≥2% and More Frequent with INBRIJA than with Placebo in Study 1 Adverse Reactions INBRIJA 84 mg
N=114
%Placebo
N=112
%Respiratory, thoracic and mediastinal disorders Cough 15 2 Sputum discolored 5 0 Nasal discharge discoloration 2 0 Oropharyngeal pain 2 0 Gastrointestinal disorders Nausea 5 3 Vomiting 3 0 Infections and infestations Upper respiratory tract infection 6 3 Nasopharyngitis 3 2 Bronchitis/pneumonia 2 0 Nervous system disorders Dyskinesia 4 1 Headache 2 0 Injury, poisoning and procedural complications Fall 3 2 Laceration 2 0 Skin abrasion 2 0 General disorders and administration site conditions Chest discomfort 2 0 Investigations Blood bilirubin increased 2 0 Red blood cell count decreased 2 0 Musculoskeletal and connective tissue disorders Pain in extremity 2 1 Psychiatric disorders Insomnia 2 1 Vascular disorders Orthostatic hypotension/blood pressure decreased 2 0 Adverse Reactions Leading to Discontinuation in Study 1
In Study 1, 6 of 114 patients (5%) in the INBRIJA 84 mg group and 3 of 112 patients (3%) in the placebo group discontinued because of adverse reactions. The most common of these adverse reactions was cough, which lead to discontinuation in 2% of patients in the INBRIJA 84 mg group and none in the placebo group.
-
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase (MAO) Inhibitors
The use of nonselective MAO inhibitors with INBRIJA is contraindicated [see Contraindications (4)] . Discontinue use of any nonselective MAO inhibitors at least two weeks prior to initiating INBRIJA.
The use of selective MAO-B inhibitors with INBRIJA may be associated with orthostatic hypotension. Monitor patients who are taking these drugs concurrently.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with the use of INBRIJA in pregnant women. In animal studies, carbidopa/levodopa has been shown to be developmentally toxic (including teratogenic effects) [see Data]. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
When administered to pregnant rabbits throughout organogenesis, carbidopa/levodopa caused both visceral and skeletal malformations in rabbits. No teratogenic effects were observed when carbidopa/levodopa was administered to pregnant mice throughout organogenesis.
There was a decrease in the number of live pups delivered by rats receiving carbidopa/levodopa during organogenesis.
8.2 Lactation
Risk Summary
The prolactin-lowering action of dopamine suggests that levodopa may interfere with lactation, although there are limited data on the effects of levodopa on milk production in lactating women.
Levodopa has been detected in human milk. There are no adequate data on the effects of levodopa on the breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for INBRIJA and any potential adverse effects on the breastfed infant from INBRIJA or from the underlying maternal condition.
8.5 Geriatric Use
Of the Parkinson's disease patients in Study 1 who took INBRIJA 84 mg, 49% (n=56) were 65 years of age and older and 51% (n=58) were under 65 years of age. Of these patients, the following age-related differences in adverse reactions were reported in patients 65 years of age and older vs. in patients under 65 years of age, respectively: cough 25% vs. 5%; upper respiratory tract infection 11% vs. 2%; nausea 7% vs. 3%; vomiting 4% vs. 2%; pain in the extremities 4% vs. 0%; and discolored nasal discharge 4% vs. 0%.
-
10 OVERDOSAGE
Based on the limited available information, the acute symptoms of carbidopa/levodopa overdosage can be expected to arise from dopaminergic overstimulation. Using more than one dose (84 mg) to treat the same OFF period may result in CNS disturbances, with an increasing risk for cardiovascular disturbance (e.g., hypotension, tachycardia) and increased risk for new or worsening psychiatric problems at higher doses.
Reports of rhabdomyolysis and transient renal insufficiency suggest that levodopa overdosage may give rise to systemic complications.
Monitor patients and provide supportive care. Patients should receive electrocardiographic monitoring for the development of arrhythmias; if needed, appropriate antiarrhythmic therapy should be given. The possibility that the patient may have taken other drugs, increasing the risk of drug interactions (especially catechol-structured drugs) should be taken into consideration.
-
11 DESCRIPTION
INBRIJA consists of a dry powder formulation of levodopa for oral inhalation with the INBRIJA inhaler. The inhalation powder is packaged in white hypromellose capsules.
Each capsule contains a spray-dried powder of 42 mg levodopa active ingredient with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and sodium chloride.
The active component of INBRIJA is levodopa, an aromatic amino acid. Its chemical name is (2S)-2-amino-3-(3,4-dihydroxyphenyl) propanoic acid and its structural formula is:
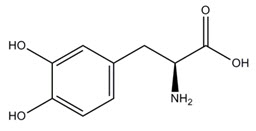
Levodopa has a molecular weight of 197.19 g/mol and molecular formula C 9H 11NO 4. Levodopa is a white to slightly off-white powder and is readily soluble in formic acid, slightly soluble in water, and practically insoluble in ethanol and diethyl ether; it dissolves in dilute hydrochloric acid.
The INBRIJA inhaler is a plastic device with a blue body, blue cap, and white mouthpiece used for inhaling INBRIJA powder.
The INBRIJA inhaler is breath-actuated by the patient. Under standardized in vitro testing conditions, the INBRIJA inhaler delivered 36.1 mg of levodopa (emitted dose) for the 42 mg capsule from the mouthpiece. No significant difference in emitted dose was observed when varying the flow rate and volume from 20 liters per minute/1L up to 90 liters per minute/2L. Peak inspiratory flow rates (PIFR) achievable through the INBRIJA inhaler were evaluated in 24 adult patients with mild to moderate Parkinson's disease. The mean PIFR was 64 L/min (range 39–98 L/min) for patients in the ON state and 57 L/min (range 29–98 L/min) in the OFF state.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Levodopa, the metabolic precursor of dopamine, crosses the blood-brain barrier and presumably is converted to dopamine in the brain. This is thought to be the mechanism whereby levodopa relieves symptoms of Parkinson's disease.
12.3 Pharmacokinetics
In the presence of carbidopa, the pharmacokinetics of levodopa are dose-proportional in healthy subjects taking up to 84 mg of INBRIJA. In the presence of carbidopa, the terminal elimination half-life (t 1/2) of levodopa following a single administration of INBRIJA 84 mg was 2.3 hours.
Absorption
After a single dose of INBRIJA 84 mg (two 42 mg capsules), the median T max for plasma levodopa was approximately 0.5 hours (range 0.17–2.00 hours). In fasted healthy volunteers the bioavailability of levodopa from INBRIJA was approximately 70% relative to immediate-release oral levodopa tablets. The dose-normalized C max of levodopa from INBRIJA is approximately 50% of that following immediate-release oral tablets.
Metabolism and Elimination
Levodopa is extensively metabolized, and the two major metabolic pathways are decarboxylation by dopa decarboxylase and O-methylation by catechol-O-methyltransferase (COMT).
Specific Populations
Geriatric Population
Clinical studies specifically designed to analyze the effects of age on the pharmacokinetics of levodopa were not conducted with INBRIJA.
Male and Female Patients
After a single dose administration of INBRIJA 84 mg, the body-weight adjusted C max and AUC 0-24 were similar between women and men. No adjustment in dosage is required based on sex.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy and safety of INBRIJA for the treatment of OFF episodes in patients with Parkinson's disease treated with oral carbidopa/levodopa was evaluated in a 12-week, randomized, placebo-controlled, double-blind study (Study 1; NCT02240030).
Study 1:
In Study 1, a total of 114 patients were treated with INBRIJA 84 mg (two 42 mg capsules), and 112 patients received placebo. Study medication could be administered up to five times a day. At baseline, patients had at least 2 hours of OFF time per day, and carbidopa/levodopa medication did not exceed 1600 mg levodopa per day. The mean UPDRS Part III scores at screening in the ON state were 14.9 for patients randomized to INBRIJA 84 mg and 16.1 for patients randomized to placebo. The UPDRS part III is designed to assess the severity of the cardinal motor findings (e.g., tremor, rigidity, bradykinesia, postural instability) in patients with Parkinson's disease.
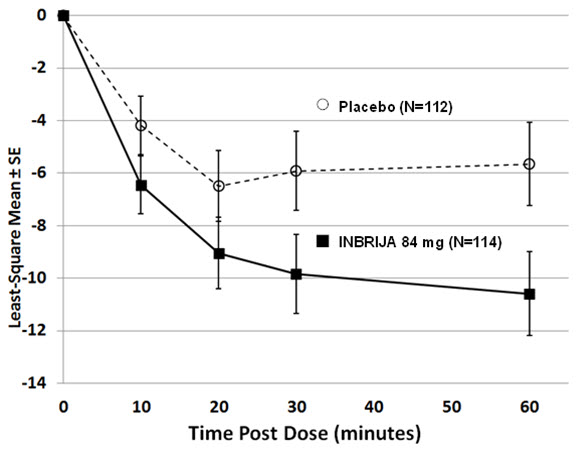
The primary endpoint was the change in Unified Parkinson's Disease Rating Scale (UPDRS) Part III motor score from pre-dose OFF state to 30 minutes post-dose, measured at Week 12. The average use of INBRIJA 84 mg or placebo was approximately 2 doses per day. At Week 12, the reduction in UPDRS Part III motor score for INBRIJA 84 mg, compared to placebo at 30 minutes post-dose, were -9.8 and -5.9, respectively (See Table 2 and Figure 1). The proportion of patients who returned to an ON state and sustained that ON through 60 minutes post-dose was 58% for INBRIJA 84 mg and 36% for placebo (p=0.003).
Table 2: Mean Change in UPDRS Part III Motor Score at 30 minutes post-dose (INBRIJA 84 mg) for the Intent-to-Treat Population at Week 12 * Treatment Pre-dose (OFF) UPDRS Part III Motor Score
(mean)Post-dose UPDRS Part III Motor Score
(mean)Mean Change 30 minutes post-dose †,‡ Difference from Placebo
(95% confidence interval)p-value - * Treatment group least-squares mean change is a model-based population estimate; the pre-dose and post-dose means are descriptive statistics.
- † Least-squares mean.
- ‡ Negative numbers indicate improvement as compared with the baseline value.
Placebo 32.1 25.3 -5.9 — — INBRIJA 84 mg 29.0 19.3 -9.8 -3.92
(-6.84, -1.00)0.009 Figure 1: Least-squares Mean Change in UPDRS Part III Motor Score After Administration of INBRIJA 84 mg vs. Placebo (at Week 12)
Study 2
The effect of INBRIJA on pulmonary function was evaluated in patients with Parkinson's disease treated with oral carbidopa/levodopa in a 12 month, randomized, controlled, open-labeled study (Study 2: NCT02352363). A total of 271 patients were treated with INBRIJA 84 mg (two 42 mg capsules), and 127 patients with Parkinson's disease in a control group were observed on their regular oral medication regimen for the treatment of Parkinson's disease. Patients with chronic obstructive pulmonary disease (COPD), asthma, or other chronic respiratory disease within the last 5 years were excluded [see Warnings and Precautions (5.6)]. Pulmonary function was assessed by spirometry every 3 months in both groups. After 12 months, the average reduction in the forced expiratory volume in 1 second (FEV 1) from baseline was the same in both groups (-0.1 L).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
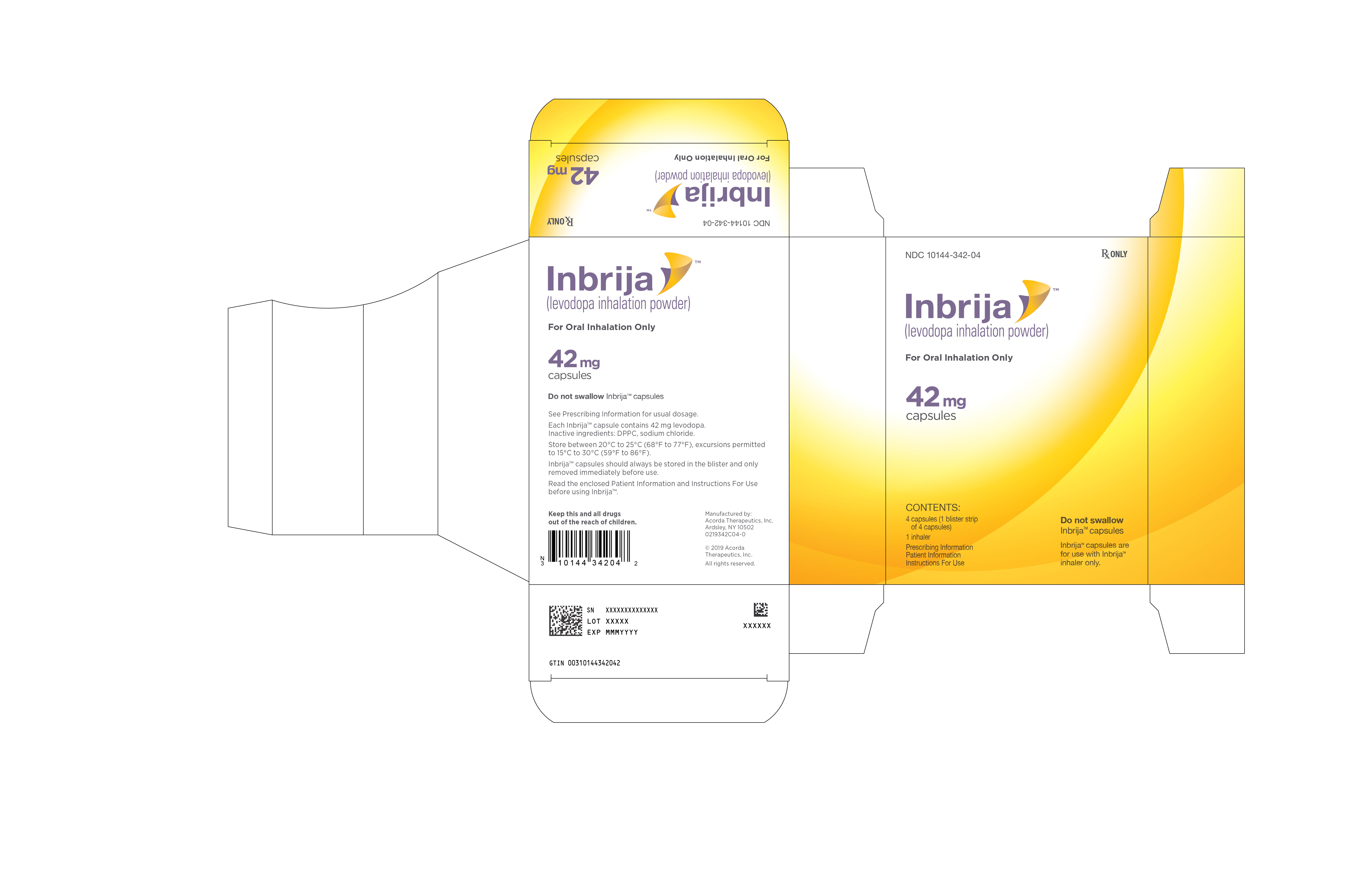
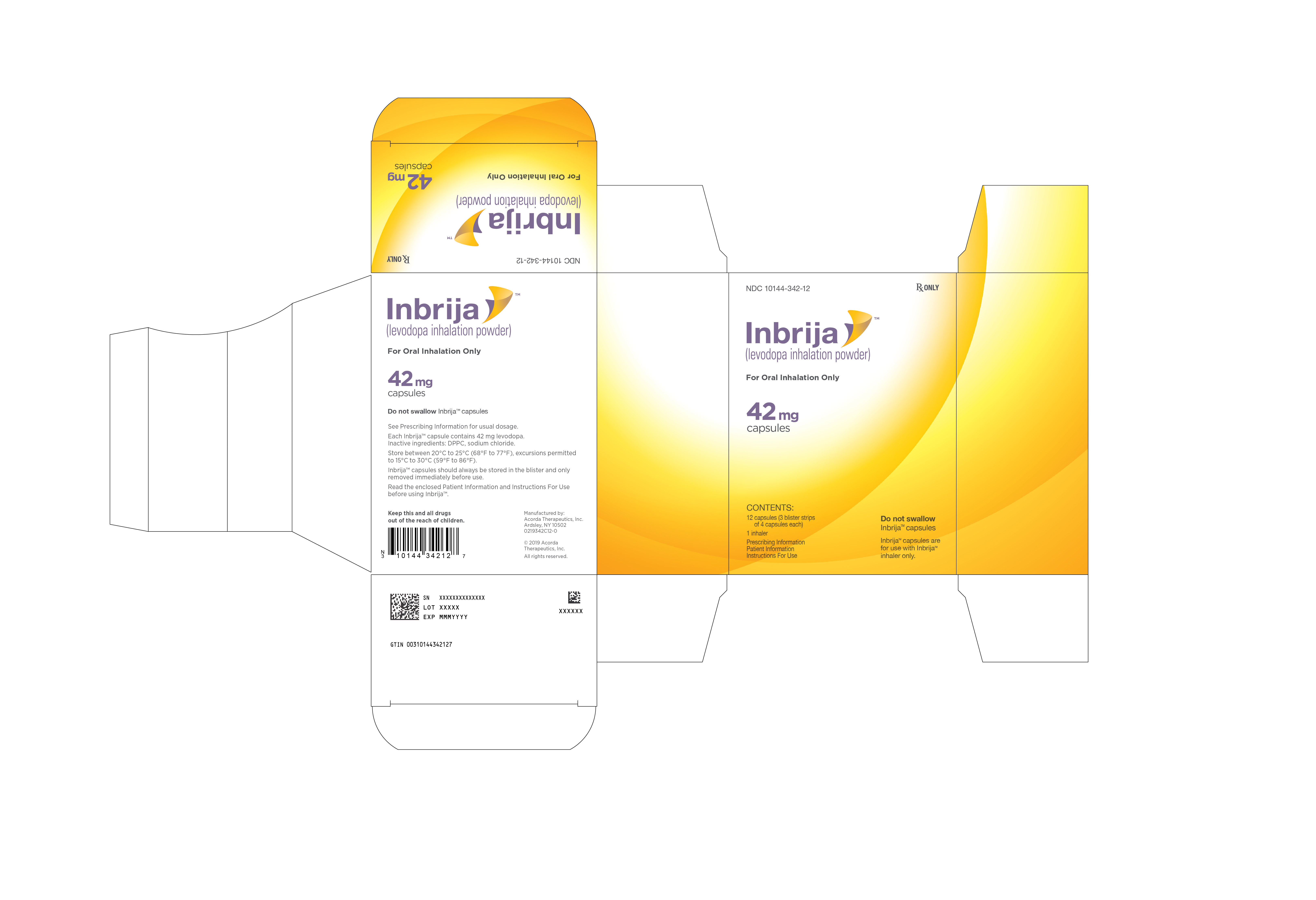
INBRIJA 42 mg contains foil blister strips of INBRIJA (levodopa inhalation powder) white capsules with two black bands on the body and "A42" in black on the cap, and one INBRIJA inhaler.
- Carton containing 4 INBRIJA capsules (1 blister card containing 4 capsules) and 1 INBRIJA inhaler: NDC: 10144-342-04
- Carton containing 12 INBRIJA capsules (3 blister cards containing 4 capsules each) and 1 INBRIJA inhaler: NDC: 10144-342-12
- Carton containing 60 INBRIJA capsules (15 blister cards containing 4 capsules each) and 1 INBRIJA inhaler: NDC: 10144-342-60
- Carton containing 92 INBRIJA capsules (23 blister cards containing 4 capsules each) and 1 INBRIJA inhaler: NDC: 10144-342-92
INBRIJA inhaler consists of a blue cap, blue handle with "INBRIJA" imprinted on it, and white mouthpiece covering the capsule chamber.
16.2 Storage and Handling
Store in a dry place between 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F).
INBRIJA capsules should always be stored in the blister packaging and only removed immediately before use. INBRIJA capsules should not be stored inside the INBRIJA inhaler.
INBRIJA capsules should be used only with the INBRIJA inhaler.
The INBRIJA inhaler should not be used to administer any other medicines.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Instructions for Administering INBRIJA
It is important for patients to understand how to correctly administer INBRIJA. It is recommended that patients be instructed in the proper administration of INBRIJA prior to use [see Dosage and Administration (2.1)] .
Patients should be counseled to take a dose of INBRIJA when the return of their Parkinson's symptoms (OFF periods) first occur [see Dosage and Administration (2.2)] .
Instruct patients to read the Instructions for Use before using INBRIJA. Remind patients that INBRIJA capsules should only be administered via the INBRIJA inhaler and the INBRIJA inhaler should not be used for administering other medications. Remind patients that the contents of INBRIJA capsules are for oral inhalation only and must not be swallowed. Instruct patients to keep INBRIJA capsules in their sealed blister packaging and to remove each INBRIJA capsule immediately before using [see Dosage and Administration (2.1)] .
Remind patients they need to orally inhale the contents of two capsules to take a full dose. They should not take more than 5 doses of INBRIJA in one day. Instruct patients they should not take more than one dose (2 capsules) per OFF period [see Dosage and Administration (2.2)] .
Lung disease
Ask patients to report whether they develop asthma, COPD, or other chronic lung diseases, since INBRIJA is not recommended in patients with these conditions [see Warnings and Precautions (5.6)] .
Cough
Inhalation of INBRIJA can lead to coughing at the time of administration [see Warnings and Precautions (5.6) and Adverse Reactions (6.1)] .
Discoloration of Body Fluids
Patients should be advised that dark color may appear in bodily fluids (saliva, sputum, urine, or sweat) when using INBRIJA [see Adverse Reactions (6.1)] .
Falling Asleep
Advise patients that certain side effects such as sleepiness and dizziness may affect some patients' ability to drive and operate machinery safely. Advise patients of the possible additive sedative effects when taking other CNS depressants in combination with INBRIJA [see Warnings and Precautions (5.1)] .
Impulse Control Disorder
Inform patients of the potential for experiencing Impulse Control Disorder: patients may experience intense urges to gamble, increased sexual urges, and other intense urges and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone, that are generally used for the treatment of Parkinson's disease [see Warnings and Precautions (5.4)] .
Dyskinesia
Instruct patients to notify their healthcare provider if abnormal involuntary movements appear or get worse during treatment with INBRIJA [see Warnings and Precautions (5.5)] .
Hypotension and Syncope
Advise patients that while they are on levodopa therapy, including INBRIJA, that they may develop orthostatic hypotension with or without symptoms such as dizziness, nausea, syncope, and sweating [see Adverse Reactions (6.1)] . Advise patients to rise slowly after sitting or lying down, especially if they have been doing so for a prolonged period.
Iron Salts
Inform patients that iron salts or multivitamins containing iron salts can reduce the bioavailability of levodopa [see Drug Interactions (7.3)].
Pregnancy and Breastfeeding
Instruct patients to notify their physicians if they become pregnant or intend to become pregnant during therapy [see Use in Specific Populations (8.1)] .
Instruct patients to notify their physicians if they intend to breastfeed or are breastfeeding an infant [see Use in Specific Populations (8.2)] .
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 09/2019 PATIENT INFORMATION
INBRIJA™ (in-BRIH-jah)
(levodopa inhalation powder)
for oral inhalation useWhat is INBRIJA?
INBRIJA is an inhaled prescription levodopa medicine used to treat the return of Parkinson's symptoms (known as OFF episodes) in people with Parkinson's disease who are treated with carbidopa-levodopa medicines. It does not replace the regular carbidopa-levodopa medicines.
It is not known if INBRIJA is safe or effective in children.Do not use INBRIJA if you: - take another medicine called a nonselective monoamine oxidase inhibitor (MAOI), such as phenelzine and tranylcypromine, or have taken a nonselective MAOI within the last 2 weeks. Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI.
Before you use INBRIJA, tell your healthcare provider about all of your medical conditions, including if you: - have asthma, chronic obstructive pulmonary disease (COPD), or any chronic lung disease.
- have daytime sleepiness from a sleep disorder or get drowsy or sleepy without warning or take a medicine to help you sleep.
- feel dizzy, nauseated, sweaty, or faint when you stand up from sitting or lying down.
- have a history of abnormal movement (dyskinesia).
- have or have had a mental health problem such as hallucinations or psychosis.
- have urges that you are unable to control (for example, gambling, increased sexual urges, intense urges to spend money, or binge eating).
- have glaucoma.
- are pregnant or plan to become pregnant. It is not known if INBRIJA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Levodopa the medicine in INBRIJA can pass into your breastmilk. It is not known if it can harm your baby.
Using INBRIJA and certain other medicines may affect each other and cause serious side effects.
Especially tell your healthcare provider if you take:- MAO-B inhibitors
- dopamine D2 receptor antagonists, including phenothiazines, butyrophenones, risperidone, and metoclopramide, or isoniazid
- iron salts or multivitamins with iron salts
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine.How should I use INBRIJA? - See the step-by-step Instructions for Use that come with your INBRIJA prescription.
- Your healthcare provider should show you the right way to use INBRIJA before you start using it.
- INBRIJA is for oral inhalation use only.
- Do not swallow INBRIJA capsules.
- Do not open INBRIJA capsules.
- Only use INBRIJA capsules with the INBRIJA inhaler. Do not use the INBRIJA inhaler to take any other medicine.
- You must be taking a daily Parkinson's disease medicine that contains carbidopa and levodopa before you start taking INBRIJA. You must not stop taking your daily Parkinson's medicine. INBRIJA does not replace your daily medicine.
- Use INBRIJA exactly as prescribed.
- The dose of INBRIJA is 2 capsules. Do not take more than 1 dose (2 capsules) for any OFF period.
- Take an INBRIJA dose as soon as you feel Parkinson's symptoms start to return.
- Do not take more than 5 doses of INBRIJA in 1 day.
What should I avoid while using INBRIJA? - Do not drive, operate machinery, or do other activities until you know how INBRIJA affects you. INBRIJA can cause sleepiness and falling asleep suddenly as late as 1 year after you start treatment.
What are the possible side effects of INBRIJA?
INBRIJA can cause serious side effects including:-
falling asleep during normal daily activities. INBRIJA may cause you to fall asleep while you are doing normal daily activities such as driving a car, doing physical tasks, using hazardous machinery, talking with other people, or eating.
- You could fall asleep without being drowsy or without warning. If you become drowsy while using INBRIJA, you should not drive or do activities where you need to be alert for your safety or the safety of others.
- Your chances of falling asleep while doing normal activities while using INBRIJA are greater if you take other medicines that cause drowsiness. Tell your healthcare provider if you take medicines that can make you sleepy such as sleep medicines, antidepressants, or antipsychotics.
- withdrawal-emergent hyperpyrexia and confusion. INBRIJA may cause a problem that can happen in people who suddenly lower their dose, stop using, or change their dose of INBRIJA. Symptoms may include:
- fever
- stiff muscles
- confusion
- changes in breathing and heartbeat
- low blood pressure. People on INBRIJA may also develop low blood pressure (hypotension) that can happen without or with the following symptoms:
- dizziness
- nausea
- fainting
- sweating
Get up slowly after sitting or lying down, especially if you have been sitting or lying down for a long time. Tell your healthcare provider if you have any of these symptoms. -
hallucinations and other psychosis. INBRIJA can cause or worsen psychotic symptoms including:
- hallucinations (seeing or hearing things that are not real)
- confusion, disorientation, or disorganized thinking
- trouble sleeping (insomnia)
- dreaming a lot
- being overly suspicious or feeling people want to harm you (paranoid ideation)
- believing things that are not real (delusional beliefs)
- acting aggressive
- feeling agitated or restless
- unusual urges. Some people using medicines like INBRIJA for Parkinson's have had unusual urges such as gambling, binge eating or eating that you cannot control (compulsive), compulsive shopping and sexual urges. If you or your family members notice that you are having unusual urges, talk to your healthcare provider.
- uncontrolled, sudden body movements (dyskinesia). INBRIJA may cause or worsen movements you cannot control in your face, tongue, or other body parts. Tell your healthcare provider if this happens. Your treatment with INBRIJA may need to be stopped or your other medicines for Parkinson's may need to be changed.
- bronchospasm. People with lung disease such as asthma, COPD, or other lung diseases have a risk of wheezing or difficulty breathing (bronchospasm) after inhaling INBRIJA. If you have these symptoms, stop taking INBRIJA and call your healthcare provider or go to the nearest hospital emergency room right away.
- increased eye pressure. INBRIJA may cause increased intraocular pressure in people with glaucoma. Your healthcare provider should check your eyes while you are using INBRIJA.
- changes in certain lab values. INBRIJA may cause changes in certain lab tests, including liver tests.
- cough
- upper respiratory tract infection
- nausea
- change in the color of your saliva or spit
How should I store INBRIJA? - Store the inhaler and capsules in a dry place at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep capsules in their foil (blister) packages until just before you are ready to use them.
- Do not store capsules inside the inhaler for a future dose.
- Keep the inhaler and capsules dry.
- Throw out the inhaler after all capsules in the carton have been used. Use the new inhaler that comes with your prescription refill.
General information about the safe and effective use of INBRIJA
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use INBRIJA for a condition for which it was not prescribed. Do not give INBRIJA to other people even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about INBRIJA that is written for health professionals.What are the ingredients in INBRIJA?
Active ingredient: levodopa
Inactive ingredients: 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), sodium chloride.
For more information, go to www.INBRIJA.com, or call 1-800-367-5109. -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
INBRIJA™ (in-BRIH-jah)
(levodopa inhalation powder)
For Oral Inhalation OnlyRead and follow these instructions before you start using INBRIJA and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important Information about using INBRIJA - Do not swallow INBRIJA capsules
- INBRIJA capsules should only be used with the INBRIJA inhaler and inhaled through your mouth (oral inhalation)
Overview:
A complete dose is 2 capsules.
You will load 1 capsule into the inhaler and breathe in (inhale). Then, you will remove the used capsule and load a second capsule into the inhaler and breathe in. Do not swallow INBRIJA capsules.
- Each carton contains 1 INBRIJA inhaler and capsules in sealed foil packages. When you open a new carton, always use the new inhaler supplied.
- Do not use capsules after the expiration date printed on the package.
- Do not load 2 capsules at the same time.
- Throw out all used capsules immediately after use.
- Throw out the inhaler after all capsules in the carton have been used.
- Make sure your hands are clean and dry when using the inhaler and capsules.
If you have any questions, ask your healthcare provider or pharmacist. If you have problems using INBRIJA, or if your INBRIJA inhaler becomes lost or damaged and you need a replacement, contact INBRIJA support at 1-800-367-5109. Then, call your healthcare provider for treatment instructions until you receive your replacement inhaler.
Parts of your INBRIJA Inhaler
(see Figure A)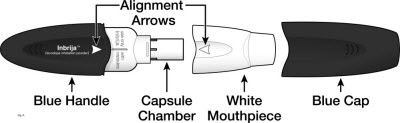
Capsules (see Figure B)
Each carton includes strips of 4 capsules.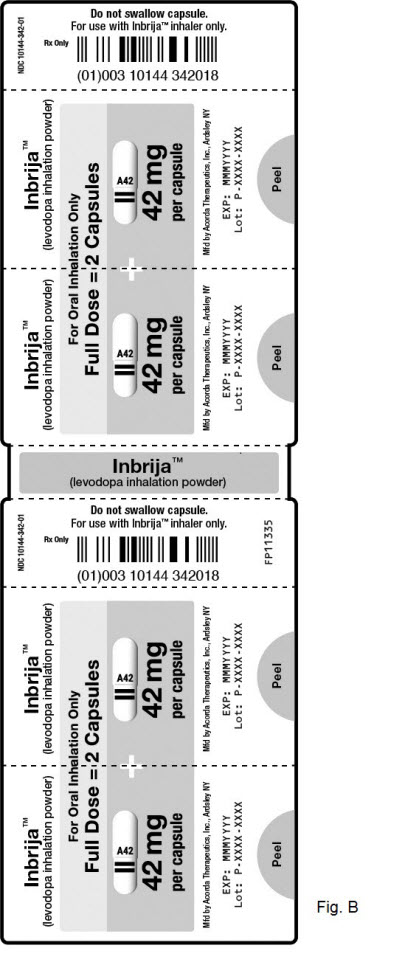
(see Figure C)
Prepare and take a total of 2 capsules.
Take each capsule 1 at a time for a full dose.
(see Figure D)
Full Dose = 2 Capsules
Prepare Your Dose
Step 1. Gather Supplies
Find a clean and dry surface.
Make sure your hands are clean and dry.
Get inhaler and strip of capsules.
Tear off package of 2 capsules (see Figure E).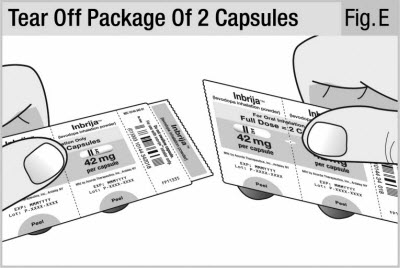
Step 2. Check Expiration
Check the expiration date on the package (see Figure F).
Step 3. Remove Blue Cap
Pull the cap straight off (see Figure G).
Place the cap to the side. You will need it later to store the inhaler.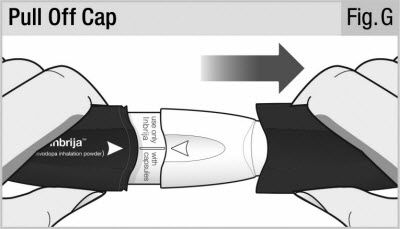
Step 4. Twist Off White Mouthpiece
Twist and pull off the mouthpiece to separate it from the handle (see Figure H).
Place the mouthpiece and inhaler on a clean and dry surface.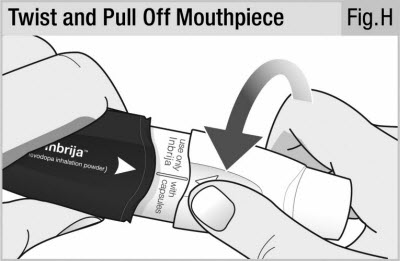
Step 5. Remove 1 Capsule From Package
Carefully peel back the foil and take out 1 capsule (see Figure I).
Do not try to push the capsule through the back of the foil package.
Only remove 1 capsule at a time, and just before use.
Do not use any capsule that looks crushed, damaged or wet. Throw it away and get a new capsule.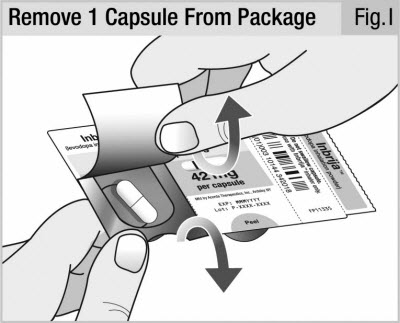
Step 6. Load Capsule
Hold the inhaler upright using the handle.
Drop 1 capsule into the opening of the capsule chamber (see Figure J).
Do not try to load 2 capsules at the same time.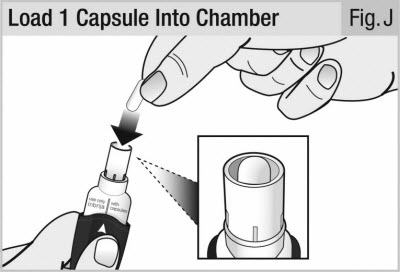
Step 7. Attach White Mouthpiece
Line up the white arrows on the handle and mouthpiece (see Figure K).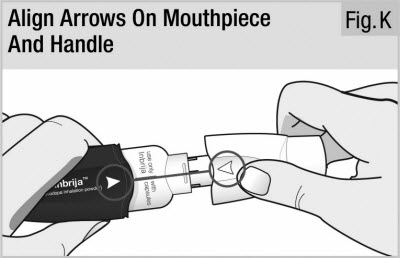
Firmly push the mouthpiece and handle together until you hear a click. This punctures the capsule (see Figure L). 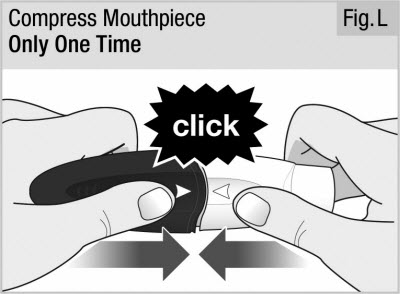
Release the mouthpiece. The mouthpiece will spring back and stay attached (see Figure M).
Your inhaler is now ready to use.
Do not push the handle and mouthpiece together more than 1 time. This may damage the capsule, and you may not get your full dose. If this happens throw the capsule away in your household trash and start over at Step 5.
Make sure the mouthpiece is securely attached and will not fall off before moving to step 8.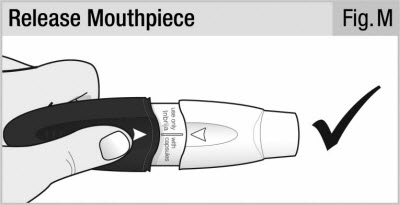
Take Your Dose Step 8. Breathe Out (Exhale)
Stand or sit with your head and chest upright.
Hold the inhaler level and away from your mouth (see Figure N).
Breathe out completely (see Figure N).
Do not breathe into mouthpiece.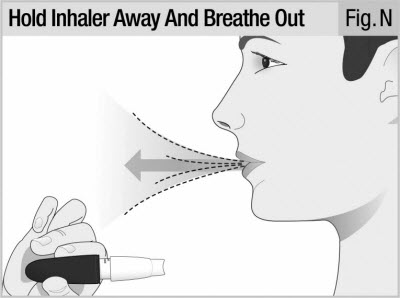
Step 9. Breathe In Deeply (Inhale)
While keeping the inhaler level, close your lips firmly around the mouthpiece (see Figure O).
Take in a deep, comfortable breath until your lungs feel full. This normally takes several seconds.
As you breathe in, you will hear and feel the capsule "whirl" (spin). The whirl means the inhaler is working and you are getting your medicine.
If you cough or stop your dose, start again from the beginning of Step 8 using the same capsule.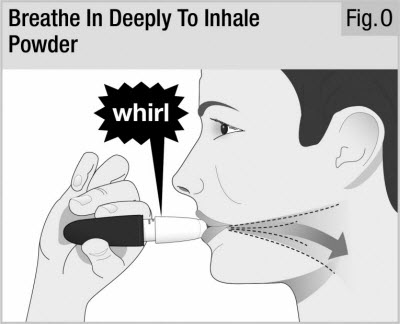
Important: If you did not hear or feel the capsule "whirl" while inhaling you may need to take a deeper, longer breath. Start again from the beginning of Step 8 using the same capsule. Step 10. Hold Breath, Then Breathe Out
Take the inhaler out of your mouth and hold your breath for 5 seconds (see Figure P).
Then breathe out.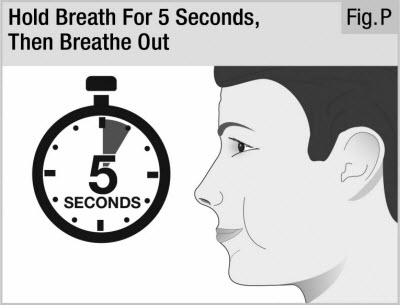
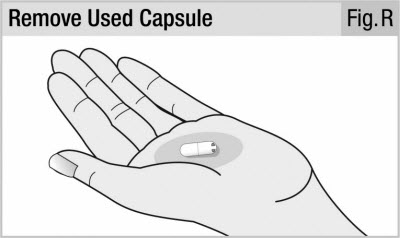
Step 11. Remove Capsule From Inhaler
Twist and pull off the mouthpiece (see Figure Q) and take out the used capsule (see Figure R).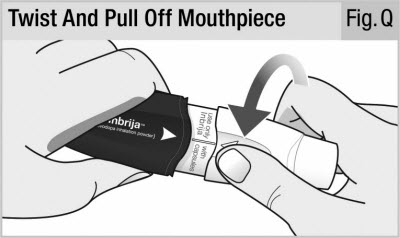
Step 12. Dose With 2 nd Capsule
Repeat Steps 5 to 11 with the second capsule to finish the full dose (see Figure S).
Dispose and Store Step 13. Throw Out Used Capsules Throw out used capsules in the household trash (see Figure T). 
Step 14. Store Inhaler Make sure there are no capsules in the inhaler before you store it.
Attach the mouthpiece to the handle by pushing until you hear a click (see Figure U).
Attach the cap over the mouthpiece (see Figure V). 
Your inhaler is now ready to store (see Figure W). 
INBRIJA Storage, Cleaning and Disposal
Storing the Inhaler and Capsules
- Store the inhaler and capsules in a dry place at room temperature from 68°F to 77°F (20 to 25°C).
- Keep capsules inside their foil (blister) packages until just before you are ready to use them.
- Do not store capsules in the inhaler for a future dose.
- Keep the inhaler and capsules dry.
- Throw out the inhaler after all capsules in the carton have been used. Use the new inhaler that comes with your prescription refill.
- Keep INBRIJA and all medicines out of reach of children.
Cleaning the Inhaler
- It is normal for some powder to remain on the inhaler.
- While cleaning the inhaler is not necessary, you may use a dry cotton swab or a dry tissue to wipe the inside or outside of the mouthpiece.
Disposing of the Inhaler and Capsules
- Throw out all used capsules in your household trash.
- After all capsules in the carton have been used, throw out the inhaler and use a new carton containing a new inhaler and capsules.
This Instructions for Use has been approved by the U.S. Food and Drug Administration Rev: 09/2019
Manufactured by:
Acorda Therapeutics, Inc.
420 Saw Mill River Road
Ardsley, NY 10502 USAInbrija™ is a trademark of Acorda Therapeutics, Inc.
©2019 Acorda Therapeutics, Inc. All rights reserved.
0919342SPL-0
-
PRINCIPAL DISPLAY PANEL - 42 mg Capsule Blister Pack Carton
NDC: 10144-342-60
Rx ONLYInbrija™
(levodopa inhalation powder)For Oral Inhalation Only
42 mg
capsulesCONTENTS:
60 capsules (15 blister
strips of 4 capsules each)
1 inhaler
Prescribing Information
Patient Information
Instructions for UseDo not swallow
Inbrija™ capsulesInbrija™ capsules are
for use with Inbrija™
inhaler only.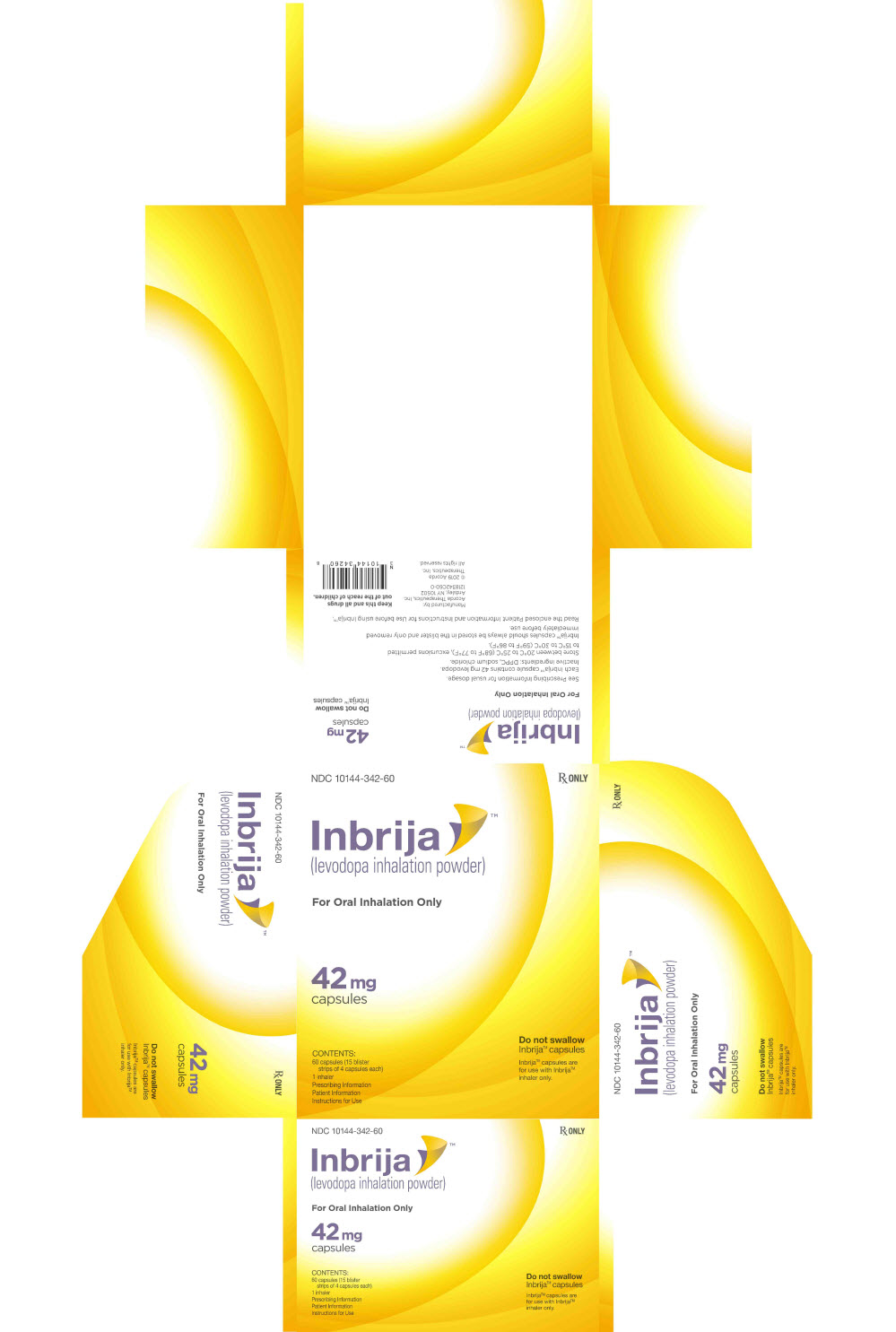
-
INGREDIENTS AND APPEARANCE
INBRIJA
levodopa capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10144-342 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVODOPA (UNII: 46627O600J) (LEVODOPA - UNII:46627O600J) LEVODOPA 42 mg Inactive Ingredients Ingredient Name Strength DIPALMITOYLPHOSPHATIDYLCHOLINE, DL- (UNII: 2W15RT5V7V) SODIUM CHLORIDE (UNII: 451W47IQ8X) Product Characteristics Color white, black Score no score Shape CAPSULE Size 23mm Flavor Imprint Code A42 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10144-342-92 23 in 1 CARTON 12/22/2018 1 NDC: 10144-342-01 4 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package 2 NDC: 10144-342-60 15 in 1 CARTON 12/22/2018 2 NDC: 10144-342-01 4 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package 3 NDC: 10144-342-32 8 in 1 CARTON 12/22/2018 3 NDC: 10144-342-01 4 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package 4 NDC: 10144-342-16 4 in 1 CARTON 12/22/2018 4 NDC: 10144-342-01 4 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package 5 NDC: 10144-342-04 1 in 1 CARTON 08/29/2019 5 NDC: 10144-342-01 4 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package 6 NDC: 10144-342-12 3 in 1 CARTON 08/29/2019 6 NDC: 10144-342-01 4 in 1 BLISTER PACK; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209184 12/22/2018 Labeler - Acorda Therapeutics, Inc. (963845136) Establishment Name Address ID/FEI Business Operations Avista Pharma Solutions, Inc. 079509111 analysis(10144-342) Establishment Name Address ID/FEI Business Operations Boston Analytical, Inc. 080408849 analysis(10144-342) Establishment Name Address ID/FEI Business Operations Sharp Corporation 143696495 label(10144-342) , pack(10144-342) Establishment Name Address ID/FEI Business Operations Ajinomoto Company, Inc. 695028225 api manufacture(10144-342) Establishment Name Address ID/FEI Business Operations Acorda Therapeutics, Inc. 963845136 manufacture(10144-342) , label(10144-342) , analysis(10144-342) Establishment Name Address ID/FEI Business Operations PPD Development Ireland Ltd. 985036175 analysis(10144-342)
Trademark Results [Inbrija]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INBRIJA 87980717 5875276 Live/Registered |
ACORDA THERAPEUTICS, INC. 2017-05-26 |
 INBRIJA 87465405 not registered Live/Pending |
ACORDA THERAPEUTICS, INC. 2017-05-26 |
 INBRIJA 87465372 not registered Live/Pending |
ACORDA THERAPEUTICS, INC. 2017-05-26 |
 INBRIJA 86717358 not registered Live/Pending |
ACORDA THERAPEUTICS, INC. 2015-08-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.