GAMUNEX-C (immune globulin- human injection
Gamunex-C by
Drug Labeling and Warnings
Gamunex-C by is a Other medication manufactured, distributed, or labeled by GRIFOLS USA, LLC, GRIFOLS THERAPEUTICS LLC, GRIFOLS BIOLOGICALS LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GAMUNEX®-C safely and effectively. See full prescribing information for GAMUNEX-C.
GAMUNEX®-C, [Immune Globulin Injection (Human), 10% Caprylate/Chromatography Purified]
Initial U.S. Approval: 2003WARNING: THROMBOSIS, RENAL DYSFUNCTION and ACUTE RENAL FAILURE
See full prescribing information for complete boxed warning.
- Thrombosis may occur with immune globulin products, including GAMUNEX-C. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity, and cardiovascular risk factors.
- For patients at risk of thrombosis, administer GAMUNEX-C at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur with immune globulin intravenous (IGIV) products in predisposed patients.
- Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. GAMUNEX-C does not contain sucrose.
- For patients at risk of renal dysfunction or failure, administer GAMUNEX-C at the minimum concentration available and the minimum infusion rate practicable. (5.2)
INDICATIONS AND USAGE
GAMUNEX-C is an immune globulin injection (human), 10% liquid indicated for treatment of:
DOSAGE AND ADMINISTRATION
Intravenous Administration Only: ITP and CIDP
Indication Dose Initial
Infusion RateMaintenance Infusion Rate
(if tolerated)ITP (2.3) 2 g/kg 1 mg/kg/min 8 mg/kg/min CIDP (2.4) loading dose
2 g/kg maintenance dose
1 g/kg2 mg/kg/min 8 mg/kg/min
Every 3 weeks- Ensure that patients with pre-existing renal insufficiency are not volume depleted; discontinue GAMUNEX-C if renal function deteriorates. (5.2)
- For patients at risk of renal dysfunction or thrombosis, administer GAMUNEX-C at the minimum infusion rate practicable. (5.2, 5.4)
Intravenous or Subcutaneous Administration: PI (2.2)
DO NOT ADMINISTER SUBCUTANEOUSLY FOR ITP PATIENTS (5.10)- * See section 2.2.
- † Adults: use up to 8 infusion sites simultaneously; pediatric: use up to 6 infusion sites simultaneously; for all ages, ensure infusion sites are at least 2 inches (5 cm) apart. (2.5)
Route of Administration Dose* Initial Infusion Rate Maintenance Infusion Rate
(if tolerated)Intravenous (IV) 300 – 600 mg/kg 1 mg/kg/min 8 mg/kg/min
Every 3 to 4 weeksSubcutaneous (SC) 1.37 x current IV dose in grams/IV
dose interval in weeksAdult:†
20 mL/hr/site
Pediatric:†
10 mL/hr/site (< 25 kg)
15 mL/hr/site (≥ 25 kg)Adult:†
20 mL/hr/site
Pediatric†
10 mL/hr/site (< 25 kg)
20 mL/hr/site (≥ 25 kg)
WeeklyDOSAGE FORMS AND STRENGTHS
GAMUNEX-C is a sterile solution for injection supplied in 1 g (10 mL), 2.5 g (25 mL), 5 g (50 mL), 10 g (100 mL), 20 g (200 mL), or 40 g (400 mL) single use vials. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- IgA deficient patients with antibodies against IgA are at greater risk of developing severe hypersensitivity and anaphylactic reactions. Have epinephrine available immediately to treat any acute severe hypersensitivity reactions. (5.1)
- Hyperproteinemia, with resultant changes in serum viscosity and electrolyte imbalances may occur in patients receiving IGIV therapy. (5.3)
- Aseptic Meningitis Syndrome (AMS) may occur, especially with high doses or rapid infusion. (5.5)
- Hemolysis, either intravascular or due to enhanced RBC sequestration, can develop subsequent to GAMUNEX-C treatments. Risk factors include high doses and non-O blood group. Closely monitor patients for hemolysis and hemolytic anemia, especially in patients with pre-existing anemia and/or cardiovascular or pulmonary compromise. (5.6)
- Monitor patients for pulmonary adverse reactions (transfusion-related acute lung injury [TRALI]). (5.7)
- Volume overload. (5.8)
- GAMUNEX-C is made from human plasma and may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. (5.9)
- GAMUNEX-C is not approved for subcutaneous use in ITP patients. Due to a potential risk of hematoma formation, do not administer GAMUNEX-C subcutaneously in patients with ITP. (5.10)
- Passive transfer of antibodies may confound serologic testing. (5.12)
ADVERSE REACTIONS
The most common adverse reactions observed in ≥ 5% patients were: (6.1)
PI: Intravenous: Cough increased, rhinitis, pharyngitis, headache, asthma, nausea, fever, diarrhea, and sinusitis.
Subcutaneous: Local infusion site reactions, fatigue, headache, upper respiratory tract infection, arthralgia, diarrhea, nausea, sinusitis, bronchitis, depression, allergic dermatitis, erythema, migraine, myalgia, viral infection, and pyrexia.ITP: Headache, ecchymosis, vomiting, fever, nausea, rash, abdominal pain, back pain and dyspepsia.
CIDP: Headache, pyrexia, hypertension, chills, rash, nausea, arthralgia, and asthenia.
To report SUSPECTED ADVERSE REACTIONS, contact Grifols Therapeutics LLC at 1-800-520-2807 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- The passive transfer of antibodies may transiently interfere with the response to live virus vaccines, such as measles, mumps and rubella. (7)
USE IN SPECIFIC POPULATIONS
- Geriatric: In patients over 65 years of age do not exceed the recommended dose, and infuse GAMUNEX-C at the minimum infusion rate practicable. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: THROMBOSIS, RENAL DYSFUNCTION, and ACUTE RENAL FAILURE
1 INDICATIONS AND USAGE
1.1 Primary Humoral Immunodeficiency (PI)
1.2 Idiopathic Thrombocytopenic Purpura (ITP)
1.3 Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
2 DOSAGE AND ADMINISTRATION
2.1 Preparation and Handling
2.2 PI
2.3 ITP
2.4 CIDP
2.5 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity Reactions to Immune Globulins
4.2 IgA Sensitive Patients with History of Hypersensitivity Reaction
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Renal Failure
5.3 Hyperproteinemia, Increased Serum Viscosity, and Hyponatremia
5.4 Thrombosis
5.5 Aseptic Meningitis Syndrome (AMS)
5.6 Hemolysis
5.7 Transfusion-related Acute Lung Injury (TRALI)
5.8 Volume Overload
5.9 Transmission of Infectious Agents
5.10 Hematoma Formation
5.11 Monitoring: Laboratory Tests
5.12 Interference with Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: THROMBOSIS, RENAL DYSFUNCTION, and ACUTE RENAL FAILURE
- Thrombosis may occur with immune globulin products, including GAMUNEX-C. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors. [see Warnings and Precautions (5.4),Patient Counseling Information (17)]
- For patients at risk of thrombosis, administer GAMUNEX-C at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. [see Dosage and Administration (2.5),Warnings and Precautions (5.4)]
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur with immune globulin intravenous (IGIV) products in predisposed patients. Patients predisposed to renal dysfunction include those with any degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs.
- Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. GAMUNEX-C does not contain sucrose.
- For patients at risk of renal dysfunction or failure, administer GAMUNEX-C at the minimum concentration available and the minimum infusion rate practicable. [see Warnings and Precautions (5.2)]
-
1 INDICATIONS AND USAGE
GAMUNEX-C is an immune globulin injection (human) 10% liquid that is indicated for the treatment of:
1.1 Primary Humoral Immunodeficiency (PI)
GAMUNEX-C is indicated for treatment of primary humoral immunodeficiency in patients 2 years of age and older. This includes, but is not limited to, congenital agammaglobulinemia, common variable immunodeficiency, X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.(1-4)
1.2 Idiopathic Thrombocytopenic Purpura (ITP)
GAMUNEX-C is indicated for the treatment of adults and children with Idiopathic Thrombocytopenic Purpura to raise platelet counts to prevent bleeding or to allow a patient with ITP to undergo surgery.(5,6)
-
2 DOSAGE AND ADMINISTRATION
2.1 Preparation and Handling
- Visually inspect GAMUNEX-C for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if turbid.
- Do not freeze. Do not use solutions that have been frozen.
- Prior to use, allow the solution to reach ambient room temperature.
- If the packaging shows any signs of tampering, do not use the product and notify Grifols Therapeutics LLC immediately [1-800-520-2807].
- The GAMUNEX-C vial is for single use only. GAMUNEX-C contains no preservative. Use any vial that has been entered promptly. Discard partially used vials. Do not store after entry into vial.
- Infuse GAMUNEX-C using a separate line by itself, without mixing with other intravenous fluids or medications the subject might be receiving.
The GAMUNEX-C infusion line can be flushed with 5% dextrose in water (D5/W) or 0.9% sodium chloride for injection. - If dilution is required, GAMUNEX-C may be diluted with 5% dextrose in water (D5/W). Do not dilute with saline.
- Content of vials may be pooled under aseptic conditions into sterile infusion bags and infused within 8 hours after pooling.
- Avoid simultaneous administration of GAMUNEX-C and Heparin through a single lumen delivery device due to GAMUNEX-C, Heparin incompatibilities. Flush Heparin Lock (Hep-Lock) through which GAMUNEX-C was administered with 5% dextrose in water (D5/W) or 0.9% sodium chloride for injection, and do not flush with Heparin. See table below.
Additional Solutions Dilution Line Flush Delivery Device Flush 5% Dextrose in water Yes Yes Yes 0.9% Sodium Chloride No Yes Yes Heparin No No No - Do not mix with immune globulin intravenous (IGIV) products from other manufacturers.
- Do not use after expiration date.
2.2 PI
As there are significant differences in the half-life of IgG among patients with primary humoral immunodeficiencies, the ideal frequency and amount of immunoglobulin therapy may vary from patient to patient. The proper amount can be determined by monitoring clinical response.
Intravenous (IV)
The dose of GAMUNEX-C for patients with PI is 300 mg/kg to 600 mg/kg body weight (3 mL/kg to 6 mL/kg) administered every 3 to 4 weeks. The dosage may be adjusted over time to achieve the desired trough levels and clinical responses.The recommended initial infusion rate is 1 mg/kg/min (0.01 mL/kg/min). If the infusion is well-tolerated, the rate may be gradually increased to a maximum of 8 mg/kg/min (0.08 mL/kg/min). For patients judged to be at risk for renal dysfunction or thrombosis, administer GAMUNEX-C at the minimum infusion rate practicable. [see Warnings and Precautions (5.2,5.4)]
If a patient routinely receives a dose of less than 400 mg/kg of GAMUNEX-C every 3 to 4 weeks (less than 4 mL/kg), and is at risk of measles exposure (i.e., traveling to a measles endemic area), administer a dose of at least 400 mg/kg (4 mL/kg) just prior to the expected measles exposure. If a patient has been exposed to measles, a dose of 400 mg/kg (4 mL/kg) should be administered as soon as possible after exposure.
Subcutaneous (SC)
The dose should be individualized based on the patient’s clinical response to GAMUNEX-C therapy and serum IgG trough levels. Begin treatment with GAMUNEX-C one week after the patient’s last IGIV infusion. See below under "Initial Weekly Dose". Prior to switching treatment from IGIV to GAMUNEX-C, obtain the patient’s serum IgG trough level to guide subsequent dose adjustments. See below under "Dose Adjustment".Establish the initial weekly dose of GAMUNEX-C by converting the monthly IGIV dose into a weekly equivalent and increasing it using a dose adjustment factor. The goal is to achieve a systemic serum IgG exposure (Area Under the Concentration-Time Curve [AUC]) not inferior to that of the previous IGIV treatment. If the patient has not been previously treated with IV GAMUNEX-C, convert the monthly IGIV dose (in grams) by multiplying by 1.37, then dividing this dose into weekly doses based on the patient’s previous IGIV treatment interval. Monitor the patient’s clinical response, and adjust dose accordingly.
Initial Weekly Dose
To calculate the initial weekly dose of subcutaneous administration of GAMUNEX-C, multiply the previous IGIV dose in grams by the dose adjustment factor of 1.37; then divide this by the number of weeks between doses during the patient’s IGIV treatment (i.e., 3 or 4).Initial SC dose (in grams) = 1.37 × previous IGIV dose (in grams)
Number of weeks between IGIV dosesTo convert the GAMUNEX-C dose (in grams) to milliliters (mL), multiply the calculated Initial SC dose (in grams) by 10.
Dose Adjustment
Over time, the dose may need to be adjusted to achieve the desired clinical response and serum IgG trough level. To determine if a dose adjustment may be considered, measure the patient’s serum IgG trough level on IGIV and as early as 5 weeks after switching from IGIV to subcutaneous. The target serum IgG trough level on weekly SC treatment is projected to be the last IGIV trough level plus 340 mg/dL. To determine if further dose adjustments are necessary, monitor the patient’s IgG trough level every 2 to 3 months.To adjust the dose based on trough levels, calculate the difference (in mg/dL) of the patient’s serum IgG trough level from the target IgG trough level (the last IGIV trough level + 340 mg/dL). Then find this difference in Table 1 and the corresponding amount (in mL) by which to increase or decrease the weekly dose based on the patient’s body weight. However, the patient’s clinical response should be the primary consideration in dose adjustment.
Table 1: Adjustment (±mL) of the Weekly Subcutaneous Dose Based on the Difference (±mg/dL) From the Target Serum IgG Trough Level - * Dose adjustment in mL is based on the slope of the serum IgG trough level response to subcutaneous administration of GAMUNEX-C dose increments (about 6.0 mg/dL per increment of 1 mg/kg per week).
Difference From
Target IgG Trough
Level (mg/dL)Body Weight (kg) 10 15 20 30 40 50 60 70 80 90 100 110 120 Dose Adjustment (mL per Week)* 50 1 1 2 3 3 4 5 6 7 8 8 9 10 100 2 3 3 5 7 8 10 12 13 15 17 18 20 150 3 4 5 8 10 13 15 18 20 23 25 28 30 200 3 5 7 10 13 17 20 23 27 30 33 37 40 250 4 6 8 13 17 21 25 29 33 38 42 46 50 300 5 8 10 15 20 25 30 35 40 45 50 55 60 350 6 9 12 18 23 29 35 41 47 53 58 64 70 400 7 10 13 20 27 33 40 47 53 60 67 73 80 450 8 11 15 23 30 38 45 53 60 68 75 83 90 500 8 13 17 25 33 42 50 58 67 75 83 92 100 For example, if a patient with a body weight of 70 kg has an actual IgG trough level of 900 mg/dL and the target level is 1,000 mg/dL, this results in a difference of 100 mg/dL. Therefore, increase the weekly dose of subcutaneous dose by 12 mL.
Monitor the patient’s clinical response, and repeat the dose adjustment as needed.
Dosage requirements for patients switching to GAMUNEX-C from another Immune Globulin Subcutaneous (IGSC) product have not been studied. If a patient on GAMUNEX-C does not maintain an adequate clinical response or a serum IgG trough level equivalent to that of the previous IGSC treatment, adjust the dose accordingly. For such patients, Table 1 also provides guidance for dose adjustment to achieve a desired IGSC trough level.
2.3 ITP
DO NOT ADMINISTER SUBCUTANEOUSLY [see Warnings and Precautions (5.10)]
GAMUNEX-C may be administered at a total dose of 2 g/kg, divided in two doses of 1 g/kg (10 mL/kg) given on two consecutive days or into five doses of 0.4 g/kg (4 mL/kg) given on five consecutive days. If after administration of the first of two daily 1 g/kg (10 mL/kg) doses, an adequate increase in the platelet count is observed at 24 hours, the second dose of 1g/kg (10 mL/kg) body weight may be withheld.
The high dose regimen (1 g/kg × 1-2 days) is not recommended for individuals with expanded fluid volumes or where fluid volume may be a concern. [see Warnings and Precautions (5.8), Clinical Studies (14)]
The recommended initial infusion rate is 1 mg/kg/min (0.01 mL/kg/min). If the infusion is well-tolerated, the rate may be gradually increased to a maximum of 8 mg/kg/min (0.08 mL/kg/min). For patients judged to be at risk for renal dysfunction or thrombosis, administer GAMUNEX-C at the minimum infusion rate practicable. [see Warnings and Precautions (5.2,5.4)]
2.4 CIDP
GAMUNEX-C may be initially administered as a total loading dose of 2 g/kg (20 mL/kg) given in divided doses over two to four consecutive days. GAMUNEX-C may be administered as a maintenance infusion of 1 g/kg (10 mL/kg) administered over 1 day or divided into two doses of 0.5 g/kg (5 mL/kg) given on two consecutive days, every 3 weeks. Not all patients may require continued maintenance therapy beyond the initial 6 months of therapy in order to maintain their therapeutic response.
The recommended initial infusion rate is 2 mg/kg/min (0.02 mL/kg/min). If the infusion is well tolerated, the rate may be gradually increased to a maximum of 8 mg/kg/min (0.08 mL/kg/min). For patients judged to be at risk for renal dysfunction or thrombosis, administer GAMUNEX-C at the minimum infusion rate practicable. [see Warnings and Precautions (5.2,5.4)]
2.5 Administration
Administer intravenously for PI, ITP and CIDP.
GAMUNEX-C may also be administered subcutaneously for the treatment of PI.
- Administer GAMUNEX-C at room temperature.
- Inspect GAMUNEX-C visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
- Do not use if turbid and/or if discoloration is observed.
Intravenous
- Use only 18 gauge needles to penetrate the stopper for dispensing product from the 10 mL vial.
- Use 16 gauge needles or dispensing pins only with 25 mL vial sizes and larger.
- Insert needles or dispensing pins only once and be within the stopper area delineated by the raised ring.
- Penetrate the stopper perpendicular to the plane of the stopper within the ring.
GAMUNEX®-C vial size Gauge of needle to penetrate stopper 10 mL 18 gauge 25, 50, 100, 200, 400 mL 16 gauge - Use promptly any vial that has been opened.
- Discard partially used vials.
- If dilution is required, GAMUNEX-C may be diluted with 5% dextrose in water (D5/W). Do not dilute with saline. Infuse GAMUNEX-C using a separate line by itself, without mixing with other intravenous fluids or medications the subject might be receiving. The GAMUNEX-C infusion line can be flushed with 5% dextrose in water (D5/W) or 0.9% sodium chloride for injection.
Subcutaneous for PI Only
Instructions for Administration
- Prior to use, allow the solution to reach ambient room temperature.
- DO NOT SHAKE.
- Do not use if the solution is cloudy or has particulates.
- Check the product expiration date on the vial. Do not use beyond the expiration date.
- Use aseptic technique when preparing and administering GAMUNEX-C for injection.
- Remove the protective cap from the vial to expose the central portion of the stopper. If the packaging shows any sign of tampering, do not use the product and notify Grifols Therapeutics LLC immediately [1-800-520-2807].
- Wipe the stopper with alcohol and allow to dry.
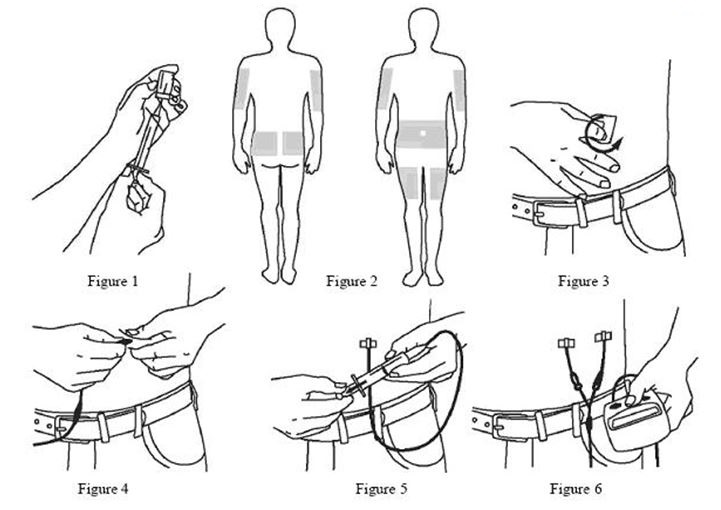
- Using a sterile syringe and needle, prepare to withdraw GAMUNEX-C by first injecting air into the vial that is equivalent to the amount of GAMUNEX-C to be withdrawn. Then withdraw the desired volume of GAMUNEX-C. If multiple vials are required to achieve the desired dose, repeat this step. (Figure 1)
- Follow the manufacturer’s instructions for filling the pump reservoir and preparing the pump, administration tubing and Y-site connection tubing, if needed. Be sure to prime the administration tubing to ensure that no air is left in the tubing or needle by filling the tubing/needle with GAMUNEX-C.
- Select the number and location of injection sites. (Figure 2)
- Cleanse the injection site(s) with antiseptic solution using a circular motion working from the center of the site and moving to the outside. Sites should be clean, dry, and at least two inches apart. (Figure 3)
- Grasp the skin between two fingers and insert the needle into the subcutaneous tissue. (Figure 4)
- After inserting each needle, make sure that a blood vessel has not been accidentally entered. Attach a sterile syringe to the end of the primed administration tubing, pull back on the plunger, and if you see blood, remove and discard the needle and administration tubing. (Figure 5)
- Repeat priming and needle insertion steps using a new needle, administration tubing and a new infusion site. Secure the needle in place by applying sterile gauze or transparent dressing over the site.
- If using multiple, simultaneous injection sites, use Y-site connection tubing and secure to the administration tubing.
- Infuse GAMUNEX-C following the manufacturer’s instructions for the pump. (Figure 6)
Rate of Administration
Intravenous
Following initial infusion (see table below), the infusion rate may be gradually increased to a maximum of 0.08 mL/kg per minute (8 mg/kg per minute) as tolerated.
Indication Initial Infusion Rate
(first 30 minutes)Maximum Infusion Rate
(if tolerated)PI 1 mg/kg/min 8 mg/kg/min ITP 1 mg/kg/min 8 mg/kg/min CIDP 2 mg/kg/min 8 mg/kg/min Monitor patient vital signs throughout the infusion. Slow or stop infusion if adverse reactions occur. If symptoms subside promptly, the infusion may be resumed at a lower rate that is comfortable for the patient.
Certain severe adverse drug reactions may be related to the rate of infusion. Slowing or stopping the infusion usually allows the symptoms to disappear promptly.
Ensure that patients with pre-existing renal insufficiency are not volume depleted. For patients at risk of renal dysfunction or thrombosis, administer GAMUNEX-C at the minimum infusion rate practicable and discontinue GAMUNEX-C if renal function deteriorates.
Subcutaneous for PI Only
For PI, it is recommended that GAMUNEX-C is infused at a rate of 20 mL per hour per infusion site for adults, and up to 8 infusion sites may be used (most patients used 4 infusion sites). Children and adolescents weighing ≥ 25 kg should start out at a slower infusion rate of 15 mL/hour/infusion site and increase their infusion rate up to 20 mL/hour/infusion site. For children and adolescents weighing < 25 kg, a rate of 10 mL/hour/infusion site is recommended. In children up to 6 infusion sites simultaneously may be used. For patients of all ages ensure that the infusion sites are at least 2 inches (5 cm) apart.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Severe hypersensitivity reactions may occur with IGIV products, including GAMUNEX-C. In case of hypersensitivity, discontinue GAMUNEX-C infusion immediately and institute appropriate treatment. Have medications such as epinephrine available for immediate treatment of acute hypersensitivity reaction.
GAMUNEX-C contains trace amounts of IgA (average 46 micrograms/mL). Patients with known antibodies to IgA may have a greater risk of developing potentially severe hypersensitivity and anaphylactic reactions. It is contraindicated in IgA deficient patients with antibodies against IgA and history of hypersensitivity reaction. [see Contraindications (4)]
5.2 Renal Failure
Acute renal dysfunction/failure, acute tubular necrosis, proximal tubular nephropathy, osmotic nephrosis and death may occur upon use of IGIV products, especially those containing sucrose.(7,8) GAMUNEX-C does not contain sucrose. Ensure that patients are not volume depleted prior to the initiation of the infusion of GAMUNEX-C. Periodic monitoring of renal function and urine output is particularly important in patients judged to have a potential increased risk for developing acute renal failure. Assess renal function, including measurement of blood urea nitrogen (BUN)/serum creatinine, prior to the initial infusion of GAMUNEX-C and again at appropriate intervals thereafter. If renal function deteriorates, consider discontinuation of GAMUNEX-C. [see Patient Counseling Information (17)] For patients judged to be at risk for developing renal dysfunction, including patients with any degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs, administer GAMUNEX-C at the minimum infusion rate practicable [less than 8 mg/kg/min (0.08 mL/kg/min)]. [see Dosage and Administration (2.5)]
5.3 Hyperproteinemia, Increased Serum Viscosity, and Hyponatremia
Hyperproteinemia, increased serum viscosity and hyponatremia may occur in patients receiving IGIV treatment, including GAMUNEX-C. It is clinically critical to distinguish true hyponatremia from a pseudohyponatremia that is associated with concomitant decreased calculated serum osmolality or elevated osmolar gap, because treatment aimed at decreasing serum free water in patients with pseudohyponatremia may lead to volume depletion, a further increase in serum viscosity and a possible predisposition to thrombosis.(9)
5.4 Thrombosis
Thrombosis may occur following treatment with immune globulin products, including GAMUNEX-C.(10-12) Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients at risk of thrombosis, administer GAMUNEX-C at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. [see Boxed Warning, Dosage and Administration (2.5), Patient Counseling Information (17)]
5.5 Aseptic Meningitis Syndrome (AMS)
AMS may occur infrequently with IGIV treatment, including GAMUNEX-C. Discontinuation of IGIV treatment has resulted in remission of AMS within several days without sequelae. The syndrome usually begins within several hours to two days following IGIV treatment. AMS is characterized by the following symptoms and signs: severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea and vomiting. Cerebrospinal fluid (CSF) studies are frequently positive with pleocytosis up to several thousand cells per cu mm, predominantly from the granulocytic series, and with elevated protein levels up to several hundred mg/dL, but negative culture results. Conduct a thorough neurological examination on patients exhibiting such symptoms and signs including CSF studies, to rule out other causes of meningitis. AMS may occur more frequently in association with high doses (2 g/kg) and/or rapid infusion of IGIV.
5.6 Hemolysis
GAMUNEX-C may contain blood group antibodies which may act as hemolysins and induce in vivo coating of red blood cells (RBCs) with immunoglobulin, causing a positive direct antiglobulin reaction and hemolysis.(13-16) Delayed hemolytic anemia can develop subsequent to IGIV therapy due to enhanced RBC sequestration, and acute hemolysis consistent with intravascular hemolysis, has been reported. [see Adverse Reactions (6)]
The following risk factors may be related to the development of hemolysis: high doses (e.g., ≥ 2 grams/kg, single administration or divided over several days) and non-O blood group.(17) Underlying inflammatory state in an individual patient may increase the risk of hemolysis, but its role is uncertain.(18)
Closely monitor patients for clinical signs and symptoms of hemolysis [see Warnings and Precautions (5.11)], particularly patients with risk factors noted above and those with pre-existing anemia and/or cardiovascular or pulmonary compromise. Consider appropriate laboratory testing in higher risk patients, including measurement of hemoglobin or hematocrit prior to infusion and within approximately 36 hours and again 7 to 10 days post infusion. If clinical signs and symptoms of hemolysis or a significant drop in hemoglobin or hematocrit have been observed, perform additional confirmatory laboratory testing. If transfusion is indicated for patients who develop hemolysis with clinically compromising anemia after receiving IGIV, perform adequate cross-matching to avoid exacerbating on-going hemolysis.
5.7 Transfusion-related Acute Lung Injury (TRALI)
Noncardiogenic pulmonary edema may occur in patients following treatment with IGIV products, including GAMUNEX-C.(19) TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Symptoms typically occur within 1 to 6 hours after treatment.
Monitor patients for pulmonary adverse reactions. [see Patient Counseling Information (17)] If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil and anti-HLA antibodies in both the product and patient serum. TRALI may be managed using oxygen therapy with adequate ventilatory support.
5.8 Volume Overload
The high dose regimen (1 g/kg x 1-2 days) is not recommended for individuals with expanded fluid volumes or where fluid volume may be a concern.
5.9 Transmission of Infectious Agents
Because GAMUNEX-C is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. No cases of transmission of viral diseases, vCJD or CJD have ever been identified for GAMUNEX-C. ALL infections suspected by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Grifols Therapeutics LLC [1-800-520-2807].
5.10 Hematoma Formation
Do not administer GAMUNEX-C subcutaneously in patients with ITP because of the risk of hematoma formation.
5.11 Monitoring: Laboratory Tests
- Periodic monitoring of renal function and urine output is particularly important in patients judged to be at increased risk of developing acute renal failure. Assess renal function, including measurement of BUN and serum creatinine, before the initial infusion of GAMUNEX-C and at appropriate intervals thereafter.
- Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies, because of the potentially increased risk of thrombosis.
- If signs and/or symptoms of hemolysis are present after an infusion of GAMUNEX-C, perform appropriate laboratory testing for confirmation.
- If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies and anti-HLA antibodies in both the product and patient’s serum.
5.12 Interference with Laboratory Tests
After infusion of IgG, the transitory rise of the various passively transferred antibodies in the patient’s blood may yield positive serological testing results, with the potential for misleading interpretation. Passive transmission of antibodies to erythrocyte antigens (e.g., A, B, and D) may cause a positive direct or indirect antiglobulin (Coombs) test.
-
6 ADVERSE REACTIONS
PI: Intravenous: The most common adverse reactions observed at a rate ≥ 5% in subjects with intravenous treatment in the clinical trials were cough increased, rhinitis, pharyngitis, headache, asthma, nausea, fever, diarrhea, and sinusitis.
PI: Subcutaneous: The most common adverse reactions observed at a rate ≥ 5% of subjects with subcutaneous treatment in the clinical trials were local infusion site reactions, fatigue, headache, upper respiratory tract infection, arthralgia, diarrhea, nausea, sinusitis, bronchitis, depression, allergic dermatitis, erythema, migraine, myalgia, viral infection, and pyrexia.
ITP: The most common adverse reactions observed at a rate ≥ 5% in subjects in the clinical trials were headache, ecchymosis, vomiting, fever, nausea, rash, abdominal pain, back pain and dyspepsia.
CIDP: The most common adverse reactions observed at a rate ≥ 5% in subjects in the clinical trial were headache, pyrexia, hypertension, chills, rash, nausea, arthralgia, and asthenia.
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of one drug cannot be directly compared to rates in other clinical trials of another drug and may not reflect the rates observed in clinical practice.
PI: Intravenous Administration
The most serious adverse event observed in clinical study subjects receiving GAMUNEX-C IV for PI was an exacerbation of autoimmune pure red cell aplasia in one subject.
In four different clinical trials to study PI, out of 157 subjects treated with GAMUNEX-C, 4 subjects discontinued due to the following adverse events: Coombs negative hypochromic anemia, autoimmune pure red cell aplasia, arthralgia/hyperhidrosis/fatigue/myalgia/nausea and migraine.
In a study of 87 subjects, 9 subjects in each treatment group were pretreated with non-steroidal medication prior to infusion, such as diphenhydramine and acetaminophen.
Table 2 lists the adverse reactions reported by at least 5% of subjects during the 9-month treatment.
Table 2: Adverse Reactions Occurring in ≥ 5% of Subjects * An adverse reaction is an adverse event that meets any of the following 3 criteria: (a) that began during or within 72 hours of the end of product infusion, (b) that was considered at least possibly related by either the investigator or the applicant, and/or (c) whose causality assessment by the investigator was missing or indeterminate. Adverse Reactions GAMUNEX®-C GAMIMUNE® N, 10% No. of subjects: 87 No. of subjects: 85 No. of subjects with adverse reaction (percentage of all subjects) No. of subjects with adverse reaction (percentage of all subjects) Cough increased 27 (31.0%) 25 (29.4%) Rhinitis 21 (24.1%) 24 (28.2%) Headache 13 (14.9%) 17 (20.0%) Pharyngitis 14 (16.1%) 16 (18.8%) Asthma 13 (14.9%) 10 (11.8%) Fever 6 (6.9%) 10 (11.8%) Nausea 10 (11.5%) 9 (10.6%) Diarrhea 6 (6.9%) 9 (10.6%) Sinusitis 5 (5.7%) 6 (7.1%) Table 3 lists the frequency of adverse reactions (as defined for Table 2), which were reported by at least 5% of subjects.
Table 3: Adverse Reactions Frequency Adverse Reactions GAMUNEX®-C GAMIMUNE® N, 10% No. of infusions: 825 No. of infusions: 865 Number (percentage of all infusions) Number (percentage of all infusions) Cough increased 40 (4.8%) 47 (5.4%) Rhinitis 34 (4.1%) 44 (5.1%) Headache 17 (2.1%) 24 (2.8%) Pharyngitis 20 (2.4%) 24 (2.8%) Fever 8 (1.0%) 20 (2.3%) Asthma 17 (2.1%) 12 (1.4%) Diarrhea 10 (1.2%) 10 (1.2%) Nausea 10 (1.2%) 10 (1.2%) Sinusitis 6 (0.7%) 7 (0.8%) The mean number of adverse reactions per infusion that occurred during or within 72 hours of the end of product infusion was 0.33 for the GAMUNEX-C and 0.39 for the GAMIMUNE® N, 10% [Immune Globulin Intravenous (Human), 10%] treatment group.
In all three trials in primary humoral immunodeficiencies, the maximum infusion rate was 0.08 mL/kg/min (8 mg/kg/min). The infusion rate was reduced for 11 of 222 exposed subjects (7 GAMUNEX-C, 4 GAMIMUNE N, 10%) at 17 occasions. In most instances, mild to moderate hives/urticaria, itching, pain or reaction at infusion site, anxiety or headache was the main reason. There was one case of severe chills. There were no anaphylactic or anaphylactoid reactions to GAMUNEX-C or GAMIMUNE N, 10% in clinical trials.
In the IV efficacy and safety study, serum samples were drawn to monitor the virus safety at baseline and one week after the first infusion of IGIV (for parvovirus B19), eight weeks after first and fifth infusion of IGIV (for hepatitis C, hepatitis B, and HIV-1), 16 weeks after the first and fifth infusion of IGIV (for hepatitis C) and at any time of premature discontinuation of the study (for hepatitis C, hepatitis B, HIV-1, and parvovirus B19). Viral markers of hepatitis C, hepatitis B, HIV-1, and parvovirus B19 were monitored by nucleic acid testing (NAT, Polymerase Chain Reaction [PCR]) and serological testing. There were no treatment related emergent findings of virus transmission for either GAMUNEX-C or GAMIMUNE N, 10%.
PI: Subcutaneous Administration (PK and Safety Studies)
Adverse reactions were divided into 2 types: 1) Local infusion site reactions, and 2) Non-infusion site adverse reactions. Table 4 lists those adverse reactions (as defined for Table 2) occurring in ≥ 2% of infusions during the SC phase of two pharmacokinetic (PK) crossover and safety trials, one in adults and adolescents and the other in children and adolescents. [see Clinical Pharmacology (12.3)]
Table 4: Most Frequent Adverse Reactions (≥ 2% of infusions) by Infusion in the SC Phase - * For each trial, rate is calculated by the total number of events divided by the number of infusions received (725 for the adult and adolescent trial and 121 for the children / adolescent trial).
- † All local infusion site reactions were a priori considered drug-related.
- ‡ At each level of summation (Preferred Term), local infusion site reactions are counted only once if they occur at the same infusion visit.
Mild – usually transient in nature and generally not interfering with normal activities
Moderate – sufficiently discomforting to interfere with normal activities
Severe – prevents normal activitiesAdverse Reactions Number (Rate*) Adult, Adolescent
(Study 060001)
Child, Adolescent
(Study T5004-401)Non-infusion Site Adverse Reactions Headache 25 (0.03) 1 (0.01) Abdominal Pain 1 (<0.01) 2 (0.02) Local Infusion Site Reactions†,‡ Mild 389 (0.54) 56 (0.46) Moderate 29 (0.04) 4 (0.03) Severe 9 (0.01) 1 (0.01) Table 5 lists the adverse reactions occurring in ≥ 5% of subjects and the frequency of adverse reactions (as defined for Table 2) per infusion.
Table 5: Most Frequent Adverse Reactions (≥ 5% of subjects) by Subject and Infusion in the SC Phase - * For each trial, rate is calculated by the total number of events divided by the number of infusions received (725 for the adult and adolescent trial and 121 for the children / adolescent trial).
- † All local infusion site reactions were a priori considered drug-related
- ‡ At each level of summation (Preferred Term), infusion site reactions are counted only once if they occur at the same infusion visit.
Adverse Reaction Adult, Adolescent
(Study 060001)
Child, Adolescent
(Study T5004-401)
No. of Subjects
n=32 (%)No. of Adverse Reactions
(Rate*)No. of Subjects
n=11 (%)No. of Adverse Reactions
(Rate*)Local Infusion Site Reaction†,‡ 24 (75.0%) 427 (0.59) 11 (100%) 61 (0.50) Fatigue 5 (15.6%) 6 (0.01) 0 0 Headache 4 (12.5%) 25 (0.03) 1 (9.1%) 1 (0.01) Upper respiratory tract infection 4 (12.5%) 5 (0.01) 1 (9.1%) 1 (0.01) Arthralgia 3 (9.4%) 6 (0.01) 0 0 Diarrhea 3 (9.4%) 6 (0.01) 0 0 Nausea 3 (9.4%) 4 (0.01) 0 0 Sinusitis 3 (9.4%) 4 (0.01) 0 0 Abdominal pain 1 (3.1%) 1 (<0.01) 1 (9.1%) 2 (0.02) Abdominal pain upper 0 0 1 (9.1%) 1 (0.01) Autoimmune thyroiditis 0 0 1 (9.1%) 1 (0.01) Drug hypersensitivity 0 0 1 (9.1%) 1 (0.01) Influenza 0 0 1 (9.1%) 1 (0.01) Oropharyngeal pain 0 0 1 (9.1%) 1 (0.01) Skin chapped 0 0 1 (9.1%) 1 (0.01) Viral upper respiratory tract infection 0 0 1 (9.1%) 1 (0.01) Wheezing 1 (3.1%) 1 (<0.01) 1 (9.1%) 1 (0.01) Bronchitis 2 (6.3%) 2 (<0.01) 0 0 Depression 2 (6.3%) 2 (<0.01) 0 0 Dermatitis allergic 2 (6.3%) 2 (<0.01) 0 0 Erythema 2 (6.3%) 2 (<0.01) 0 0 Migraine 2 (6.3%) 2 (<0.01) 0 0 Myalgia 2 (6.3%) 2 (<0.01) 0 0 Pyrexia 2 (6.3%) 2 (<0.01) 0 0 Viral infection 2 (6.3%) 2 (<0.01) 0 0 There were no serious bacterial infections in the SC phase of the PK and safety trials.
Local Infusion Site Reactions
Local infusion site reactions with SC GAMUNEX-C consisted of erythema, pain and swelling. One child discontinued due to infusion site pain. The majority of local infusion site reactions resolved within 3 days. The number of subjects experiencing an infusion site reaction and the number of infusion site reactions decreased over time as subjects received continued weekly SC infusions. At the beginning of the SC phase (week 1) in the adult and adolescent trial, a rate of approximately 1 infusion site reaction per infusion was reported, whereas at the end of the study (week 24) this rate was reduced to 0.5 infusion site reactions per infusion, a reduction of 50%. In the children and adolescent trial, the rate of local infusion site reactions decreased from week 1 for all age groups by the end of the study.
ITP
In two different clinical trials to study ITP, out of 76 subjects treated with GAMUNEX-C, 2 subjects discontinued due to the following adverse reactions: Hives and Headache/Fever/Vomiting.
One subject, a 10-year-old boy, died suddenly from myocarditis 50 days after his second infusion of GAMUNEX-C. The death was judged to be unrelated to GAMUNEX-C.
No pre-medication with corticosteroids was permitted by the protocol. Twelve ITP subjects treated in each treatment group were pretreated with medication prior to infusion. Generally, diphenhydramine and/or acetaminophen were used. More than 90% of the observed drug related adverse events were of mild to moderate severity and of transient nature.
The infusion rate was reduced for 4 of the 97 exposed subjects (1 GAMUNEX-C, 3 GAMIMUNE N, 10%) on 4 occasions. Mild to moderate headache, nausea, and fever were the reported reasons.
Table 6 lists the adverse reactions (as defined for Table 2) reported by at least 5% of subjects during the 3-month efficacy and safety study.
Serum samples were drawn to monitor the virus safety of the ITP subjects at baseline, nine days after the first infusion (for parvovirus B19), and 3 months after the first infusion of IGIV and at any time of premature discontinuation of the study. Viral markers of hepatitis C, hepatitis B, HIV-1, and parvovirus B19 were monitored by nucleic acid testing (NAT, PCR), and serological testing. There were no treatment related emergent findings of virus transmission for either GAMUNEX-C or GAMIMUNE N, 10%.
CIDP
In the CIDP efficacy and safety study, 113 subjects were exposed to GAMUNEX-C and 95 were exposed to Placebo. [see Clinical Studies (14)] As a result of the study design, the drug exposure with GAMUNEX-C was almost twice that of Placebo, with 1096 GAMUNEX-C infusions versus 575 Placebo infusions. Therefore, adverse reactions are reported per infusion (represented as frequency) to correct for differences in drug exposure between the 2 groups. The majority of loading-doses were administered over 2 days. The majority of maintenance-doses were administered over 1 day. Infusions were administered in the mean over 2.7 hours.
Table 7 shows the numbers of subjects per treatment group in the CIDP clinical trial, and the reason for discontinuation due to adverse events.
Table 7: Reasons for Discontinuation Due to Adverse Events Number of Subjects Number of Subjects Discontinued
due to Adverse EventsAdverse Event GAMUNEX®-C 113 3 (2.7%) Urticaria, Dyspnea,
BronchopneumoniaPlacebo 95 2 (2.1%) Cerebrovascular Accident,
Deep Vein ThrombosisThe most common adverse reactions with GAMUNEX-C were headache and pyrexia. Table 8 lists adverse reactions (as defined for Table 2) reported by at least 5% of subjects in any treatment group.
Table 8: Adverse Reactions Occurring in ≥ 5% of Subjects - * Reported in ≥ 5% of subjects in any treatment group.
- † Calculated by the total number of adverse reactions divided by the number of infusions received (1096 for GAMUNEX-C and 575 for Placebo).
GAMUNEX®-C
No. of subjects: 113Placebo
No. of subjects: 95MedDRA
Preferred Term*No. of
Subjects
(%)No. of
Adverse
ReactionsIncidence
density†No. of
Subjects
(%)No. of
Adverse
ReactionsIncidence
density†Headache 35 (31.0%) 50 0.046 7 (7.4%) 9 0.016 Pyrexia 15 (13.3%) 27 0.025 0 0 Hypertension 10 (8.8%) 19 0.017 3 (3.2%) 3 0.005 Chills 9 (8.0%) 10 0.009 0 0 Nausea 7 (6.2%) 9 0.008 3 (3.2%) 3 0.005 Rash 7 (6.2%) 10 0.009 1 (1.1%) 1 0.002 Arthralgia 6 (5.3%) 7 0.006 0 0 Asthenia 6 (5.3%) 6 0.005 1 (1.1%) 2 0.003 The most serious adverse reaction observed in clinical study subjects receiving GAMUNEX-C for CIDP was pulmonary embolism (PE) in one subject with a history of PE.
Laboratory Abnormalities
During the course of the clinical program, ALT and AST elevations were identified in some subjects.
- For ALT, in the IV PI study treatment emergent elevations above the upper limit of normal were transient and observed among 14/80 (18%) of subjects in the GAMUNEX-C group versus 5/88 (6%) of subjects in the GAMIMUNE N, 10% group (p = 0.026).
- In the SC PI study treatment emergent laboratory abnormalities during the SC phase occurred in several subjects. Four subjects (4/32, 13%) had elevated Alkaline Phosphatase. One subject (1/32, 3%) had an elevated ALT and three subjects (3/32, 9%) had an elevated AST. No elevations were > 1.6 times the upper limit of normal.
- In the ITP study which employed a higher dose per infusion, but a maximum of only two infusions, the reverse finding for elevation of ALT was observed among 3/44 (7%) of subjects in the GAMUNEX-C group versus 8/43 (19%) of subjects in the GAMIMUNE N, 10% group (p = 0.118).
- In the CIDP study, 15/113 (13%) of subjects in the GAMUNEX-C group and 7/95 (7%) in the Placebo group (p=0.168) had a treatment emergent transient elevation of ALT.
Elevations of ALT and AST were generally mild (< 3 times upper limit of normal), transient, and were not associated with obvious symptoms of liver dysfunction.
GAMUNEX-C may contain low levels of anti-Blood Group A and B antibodies primarily of the IgG4 class. Direct antiglobulin tests (DAT or direct Coombs tests), which are carried out in some centers as a safety check prior to red blood cell transfusions, may become positive temporarily. There were 2 cases of hemolytic anemia across these clinical trials. One hemolytic event not associated with positive DAT findings was observed in the IV PI study in a woman with common variable immune deficiency and B12 deficiency (pernicious anemia) at a dose of (450 mg/kg). The other hemolytic event occurred in the CIDP study in a subject with positive DAT at a dose of 1g/kg.
6.2 Postmarketing Experience
Because adverse reactions are voluntarily reported post-approval from a population of uncertain size, it is not always possible to reliably estimate their frequencies or establish a causal relationship to product exposure.
The following adverse reactions have been identified during post-approval use of IGIV products, (8,20) including GAMUNEX-C:
- Infusion Reactions: Hypersensitivity (e.g., anaphylaxis), tachycardia, malaise, flushing, or other skin reactions, chest discomfort, rigors, and changes in blood pressure
- Renal: Acute renal dysfunction/failure, osmotic nephropathy
- Respiratory: Apnea, Acute Respiratory Distress Syndrome (ARDS), TRALI, cyanosis, hypoxemia, pulmonary edema, bronchospasm
- Cardiovascular: Cardiac arrest, thromboembolism, vascular collapse, hypotension
- Neurological: Coma, loss of consciousness, seizures/convulsions, tremor, aseptic meningitis
- Integumentary: Stevens-Johnson syndrome, epidermolysis, erythema multiforme, dermatitis (e.g., bullous dermatitis)
- Hematologic: Pancytopenia, leukopenia, hemolysis, hemolytic anemia, positive direct antiglobulin (Coombs test)
- General/Body as a Whole: Rigors
- Gastrointestinal: Hepatic dysfunction
-
7 DRUG INTERACTIONS
GAMUNEX-C may be diluted with 5% dextrose in water (D5/W). Do not dilute with saline. Admixtures of GAMUNEX-C with other drugs and intravenous solutions have not been evaluated. It is recommended that GAMUNEX-C be administered separately from other drugs or medications which the patient may be receiving. The product should not be mixed with IGIVs from other manufacturers.
The infusion line may be flushed before and after administration of GAMUNEX-C with 5% dextrose in water (D5/W) or 0.9% sodium chloride for injection.
Avoid simultaneous administration of GAMUNEX-C and Heparin through a single lumen delivery device due to GAMUNEX-C, Heparin incompatibilities. Flush Heparin Lock (Hep-Lock) through which GAMUNEX-C was administered with 5% dextrose in water (D5/W) or 0.9% sodium chloride for injection, and do not flush with Heparin.
Various passively transferred antibodies in immunoglobulin preparations can confound the results of serological testing.
Passive transfer of antibodies may transiently interfere with the immune response to live virus vaccines such as measles, mumps, rubella and varicella. Inform the immunizing physician of recent therapy with GAMUNEX-C so that appropriate measures may be taken. [see Patient Counseling Information (17)]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with GAMUNEX-C use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with GAMUNEX-C. It is not known whether GAMUNEX-C can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. GAMUNEX-C should be given to a pregnant woman only if clearly needed. In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of GAMUNEX-C in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for GAMUNEX-C and any potential adverse effects on the breastfed infant from GAMUNEX-C or from the underlying maternal condition.
8.4 Pediatric Use
PI: Intravenous
GAMUNEX-C was evaluated in 18 pediatric subjects (age range 0-16 years). Twenty-one percent of PI subjects exposed to GAMUNEX-C were children. Pharmacokinetics, safety and efficacy were similar to those in adults with the exception that vomiting was more frequently reported in pediatrics (3 of 18 subjects). No pediatric-specific dose requirements were necessary to achieve serum IgG levels.
PI: Subcutaneous
SC GAMUNEX-C was evaluated in three pediatric subjects (age range 13-15 years) with PI along with adults, and separately in a second trial in 11 children and adolescents (age range 2-16 years). Pharmacokinetics and safety were similar to those in adults. No pediatric-specific dose requirements were necessary to achieve circulating IgG levels. Efficacy and safety in pediatric patients under 2 years of age using the SC route of administration have not been established.
ITP
For treatment of ITP, GAMUNEX-C must be administered by the intravenous route.
GAMUNEX-C was evaluated in 12 pediatric subjects with acute ITP. Twenty-five percent of the acute ITP subjects exposed to GAMUNEX-C were children. Pharmacokinetics, safety and efficacy were similar to those in adults with the exception that fever was more frequently reported in pediatrics (6 of 12 subjects). No pediatric-specific dose requirements were necessary to achieve serum IgG levels. One subject, a 10-year-old boy, died suddenly from myocarditis 50 days after his second infusion of GAMUNEX-C. The death was judged to be unrelated to GAMUNEX-C.
CIDP
The safety and effectiveness of GAMUNEX-C have not been established in pediatric subjects with CIDP.
8.5 Geriatric Use
Use caution when administering GAMUNEX-C to patients age 65 and over who are at increased risk for thrombosis or renal insufficiency. [see Boxed Warning, Warnings and Precautions (5.2, 5.4)] Do not exceed recommended doses, and administer GAMUNEX-C at the minimum infusion rate practicable. Clinical studies of GAMUNEX-C did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
- 10 OVERDOSAGE
-
11 DESCRIPTION
GAMUNEX-C is a ready-to-use sterile, non-pyrogenic solution of human immune globulin protein for intravenous and subcutaneous (PI indication only) administration. GAMUNEX-C is clear to opalescent, and colorless to pale yellow. GAMUNEX-C consists of 9%–11% protein in 0.16–0.24 M glycine. Not less than 98% of the protein has the electrophoretic mobility of gamma globulin. The main component of GAMUNEX-C is IgG (≥ 98%) with a sub-class distribution of IgG1, IgG2, IgG3 and IgG4 of approximately 62.8%, 29.7%, 4.8% and 2.7% respectively. The distribution of IgG subclasses is similar to that found in normal serum.
GAMUNEX-C contains trace levels of fragments, IgA (average 0.046 mg/mL), and IgM. GAMUNEX-C doses of 1 g/kg correspond to a glycine dose of 0.15 g/kg. While toxic effects of glycine administration have been reported, the doses and rates of administration were 3–4 fold greater than those for GAMUNEX-C. In another study it was demonstrated that intravenous bolus doses of 0.44 g/kg glycine were not associated with serious adverse effects.(21) Caprylate is a saturated medium-chain (C8) fatty acid of plant origin. Medium chain fatty acids are considered to be essentially non-toxic. Human subjects receiving medium chain fatty acids parenterally have tolerated doses of 3.0 to 9.0 g/kg/day for periods of several months without adverse effects.(22) Residual caprylate concentrations in the final container are no more than 0.216 g/L (1.3 mmol/L). The measured buffer capacity is 35 mEq/L (0.35 mEq/g protein) and the osmolality is 258 mOsmol/kg solvent, which is close to physiological osmolality (285-295 mOsmol/kg). A dose of 1 g/kg body weight therefore represents an acid load of 0.35 mEq/kg body weight. The total buffering capacity of whole blood in a normal individual is 45–50 mEq/L of blood, or 3.6 mEq/kg body weight. Thus, the acid load delivered with a dose of 1 g/kg of GAMUNEX-C would be neutralized by the buffering capacity of whole blood alone, even if the dose was infused instantaneously. The pH of GAMUNEX-C is 4.0–4.5. GAMUNEX-C contains no preservative. GAMUNEX-C is not made with natural rubber latex.
GAMUNEX-C is made from large pools of human plasma by a combination of cold ethanol fractionation, caprylate precipitation and filtration, and anion-exchange chromatography. Isotonicity is achieved by the addition of glycine. GAMUNEX-C is incubated in the final container (at the low pH of 4.0–4.3). The product is intended for intravenous administration and may be administered subcutaneously in treatment of PI.
The capacity of the manufacturing process to remove and/or inactivate enveloped and non-enveloped viruses has been validated by laboratory spiking studies on a scaled down process model, using the following enveloped and non-enveloped viruses: human immunodeficiency virus, type I (HIV-1) as the relevant virus for HIV-1 and HIV–2; bovine viral diarrhea virus (BVDV) as a model for hepatitis C virus; pseudorabies virus (PRV) as a model for large enveloped DNA viruses (e.g., herpes viruses); Reovirus type 3 (Reo) as a model for non-enveloped viruses and for its resistance to physical and chemical inactivation; hepatitis A virus (HAV) as relevant non-enveloped virus, and porcine parvovirus (PPV) as a model for human parvovirus B19.(23)
Overall virus reduction was calculated only from steps that were mechanistically independent from each other and truly additive. In addition, each step was verified to provide robust virus reduction across the production range for key operating parameters.
Table 9: Log10 Virus Reduction - * C/I - Interference by caprylate precluded determination of virus reduction for this step. Although removal of viruses is likely to occur at the caprylate precipitation/depth filtration step, BVDV is the only enveloped virus for which reduction is claimed. The presence of caprylate prevents detection of other, less resistant enveloped viruses and therefore their removal cannot be assessed.
- † NA - Not Applicable: This step has no effect on non-enveloped viruses.
- ‡ Some mechanistic overlap occurs between depth filtration and other steps. Therefore, Grifols Therapeutics LLC has chosen to exclude this step from the global virus reduction calculations.
- § CAP - The presence of caprylate in the process at this step prevents detection of enveloped viruses, and their removal cannot be assessed.
- ¶ M/I - Interference by the process intermediate matrix precluded determination of virus removal capacity for this step.
- # Sum of reduction factors greater than or equal to 1 log10.
Process Step Log10 Virus Reduction Enveloped Viruses Non-enveloped Viruses HIV PRV BVDV Reo HAV PPV Caprylate Precipitation/Depth Filtration C/I* C/I* 2.7 ≥ 3.5 ≥ 3.6 4.0 Caprylate Incubation ≥ 4.5 ≥ 4.6 ≥ 4.5 NA† NA† NA† Depth Filtration‡ CAP§ CAP§ CAP§ ≥ 4.3 ≥ 2.0 3.3 Column Chromatography ≥ 3.0 ≥ 3.3 4.0 ≥ 4.0 ≥ 1.4 4.2 Nanofiltration ≥ 3.7 M/I¶ ≥ 4.1 ≥ 1.8 M/I¶ < 1.0 Low pH Incubation ≥ 6.5 ≥ 4.3 ≥ 5.1 NA† NA† NA† Global Reduction# ≥ 17.7 ≥ 12.2 ≥ 20.4 ≥ 9.3 ≥ 5.0 8.2 Additionally, the manufacturing process was investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the vCJD and CJD agents.(23)
Several of the individual production steps in the GAMUNEX-C manufacturing process have been shown to decrease TSE infectivity of that experimental model agent. TSE reduction steps include two depth filtrations (in sequence, a total of ≥ 6.6 log10). These studies provide reasonable assurance that low levels of CJD/vCJD agent infectivity, if present in the starting material, would be removed.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
PI
GAMUNEX-C supplies a broad spectrum of opsonic and neutralizing IgG antibodies against bacterial, viral, parasitic, and mycoplasmal agents, and their toxins. The mechanism of action in PI has not been fully elucidated.
ITP
The mechanism of action of high doses of immunoglobulins in the treatment of ITP has not been fully elucidated.
CIDP
The precise mechanism of action in CIDP has not been fully elucidated.
12.3 Pharmacokinetics
Two pharmacokinetic crossover trials were carried out with GAMUNEX-C in 44 subjects with Primary Humoral Immunodeficiency to assess intravenous vs subcutaneous administration. In the first study, a single sequence, open-label, crossover trial in adults and adolescents, the pharmacokinetics, safety, and tolerability of SC administered GAMUNEX-C in subjects with PI were evaluated.(24) A total of 32 and 26 subjects received GAMUNEX-C as IV or SC for PK study, respectively, of whom 3 were adolescents. Subjects received GAMUNEX-C 200-600 mg/kg IV every 3-4 weeks for at least 3 months, at which time they entered the IV phase of the study. Subjects were crossed over to weekly SC infusions. The weekly SC dose was determined by multiplying the total IV dose by 1.37 and dividing the resultant new total dose by 3 or 4 depending on the previous IV interval.
In the second study, a single sequence, open-label, crossover trial, the pharmacokinetics, safety, and tolerability of SC administered GAMUNEX-C were evaluated in children and adolescents. The design of the study was essentially the same as above. A total of 11 subjects received GAMUNEX-C as IV and 10 received GAMUNEX-C as SC for PK analysis. Age groups were as follows: age 2 to 5 years, [N = 1 both phases]; 6 to 11 years, [N = 5 completing IV phase, N = 4 evaluated in SC phase]; 12-16 years: [N = 5 both phases].
Intravenous Administration
The pharmacokinetic parameters of GAMUNEX-C, measured as total IgG for intravenous administration are shown in Table 10.
Table 10: PK Parameters Following IV Administration of GAMUNEX®-C by Age - * SD - standard deviation; NA - not applicable.
Source: studies 060001, T5004-401Age Group Statistics t1/2
(hr)AUC(0-t)
(hr*mg/mL)AUC(0-tau)
(hr*mg/mL)CL(0-t)
(mL/hr/kg)Vss
(mL/kg)2 – 5 years
N = 1Mean 1038.50 7479.0 7499.0 0.05430 82.040 SD* NA* NA* NA* NA* NA* 6 – 11 years
N = 5Mean 758.52 5953.6 6052.6 0.09128 94.784 SD* 137.989 1573.84 1333.59 0.027465 17.6773 12 – 16 years
N = 8Mean 717.90 8131.9 8009.5 0.07029 73.303 SD* 170.141 1173.38 1358.76 0.015912 17.2204 ≥ 17 years
N = 29Mean 720.62 7564.9 7524.8 0.06243 65.494 SD* 130.864 1190.68 1183.05 0.015547 18.7172 PI: Subcutaneous Administration
The PK parameter (AUC of total IgG) following IV and SC administration is summarized in Table 11 for subcutaneous vs intravenous administration in the two pharmacokinetic trials. In the adult and adolescent trial, the lower bound of the 90% confidence interval for the geometric mean ratio of AUC (SC vs. IV) was 0.861, therefore, meeting the pre-specified non-inferiority margin between the two modes of administration. The PK analysis results in children and adolescents are consistent to those in the adult and adolescent trial, demonstrating the appropriateness of the conversion factor of 1.37 applied to calculating the SC dose from the IV dose of GAMUNEX-C in pediatric populations.
Table 11: Summary of AUC of Total IgG at Steady State Following IV or SC Administration of GAMUNEX®-C by Age Route of Administration IV (N = 43) SC (N = 36) AUC Ratio,
SC/IVAge Group (N) Statistics AUC0-τ,IV
(h*mg/mL)
(0-21 days)AUC0-τ,IV
(h*mg/mL)
(0-28 days)Adj._AUC0-τ,IV*
(h*mg/mL)
(0-7 days)AUC0-τ,SC
(h*mg/mL)
(0-7 days)- * Adj._AUC0-τ,IV: Adjusted weekly IV AUC(0-7 days) is calculated as AUC(0-21 days)/3 or AUC(0-28 days)/4.
- † NC - not calculated
Source: Studies 060001, T5004-4012-5 years (N) 1 1 1 1 Mean NC† 7498.7 1874.7 2023.0 1.080 %CV NC† NC† NC† NC† - Range NC† NC† NC† NC† NC† 6-11 years (N) 5 5 4 4 Mean 6052.7 NC† 2017.6 2389.2 1.135 %CV 22% NC† 22% 19% - Range 4868 - 8308 NC† 1623 - 2769 1971 - 3039 1.10 - 1.21 12-16 years (N) 5 3 8 7 7 Mean 7396.0 9032.0 2387.6 2361.9 0.982 %CV 17% 9% 15% 14% - Range 5271 - 8754 8504 - 9950 1757 - 2918 1876 - 2672 0.86 - 1.07 ≥ 17 years (N) 10 19 29 24 24 Mean 7424.7 7577.4 2094.5 1899.9 0.882 %CV 14% 17% 20% 20% - Range 5781 - 9552 5616 - 10400 1404 - 3184 1300 - 2758 0.70 - 1.04 The mean trough concentrations (mean Ctrough) of total IgG following IV and SC administration are presented in Table 12 for both studies.
Table 12: Mean Trough Concentrations of Total IgG (mg/mL) * Measured in plasma; † Measured in serum Adult, Adolescent* Child, Adolescent† IV
Mean CtroughSC
Mean CtroughIV
Mean CtroughSC
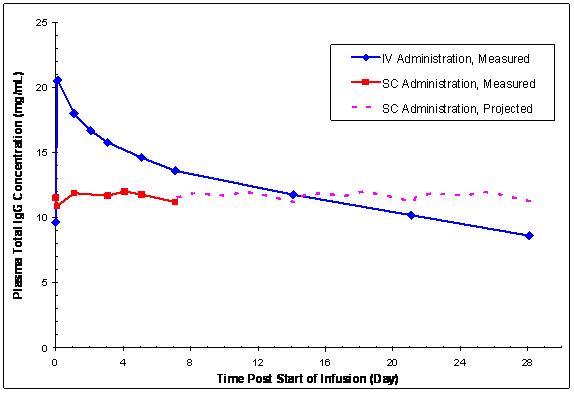
Mean Ctroughn 32 28 11 10 Mean (mg/mL) 9.58 11.4 9.97 13.25 %CV 22.3 20.4 19 14 Range 6.66-14.0 8.10-16.2 7.84-13.20 10.77-16.90 In contrast to plasma total IgG levels observed with monthly IV GAMUNEX-C treatment (rapid peaks followed by a slow decline), the plasma IgG levels in subjects receiving weekly SC GAMUNEX-C therapy were relatively stable (Figure 7, adult and adolescent trial).
Figure 7: Mean Steady-state Plasma Total IgG Concentration vs. Time Curves Following IV Administration or Weekly SC Administration in Adults and Adolescents
- * SD - standard deviation; NA - not applicable.
-
14 CLINICAL STUDIES
PI: Intravenous Administration
In a randomized, double-blind, parallel group clinical trial with 172 subjects with primary humoral immunodeficiencies GAMUNEX-C was demonstrated to be at least as efficacious as GAMIMUNE N, 10% in the prevention of any infection, i.e., validated plus clinically defined, non-validated infections of any organ system, during a nine month treatment period.(25) Twenty-six subjects were excluded from the Per Protocol analysis (2 due to non-compliance and 24 due to protocol violations). The analysis for efficacy was based on the annual rate of bacterial infections, pneumonia, acute sinusitis and acute exacerbations of chronic sinusitis.
Table 13: Efficacy Results Per Protocol Analysis - * Validated infections plus clinically defined, non-validated infections.
No. of Subjects with at Least One Infection (%) GAMUNEX®-C
(n=73)GAMIMUNE® N, 10%
(n=73)Mean Difference
(90% Confidence Interval)p-Value Validated Infections 9 (12%) 17 (23%) -0.117
(-0.220, -0.015)0.06 Acute Sinusitis 4 (5%) 10 (14%) Exacerbation of
Chronic Sinusitis5 (7%) 6 (8%) Pneumonia 0 (0%) 2 (3%) Any Infection* 56 (77%) 57 (78%) -0.020
(-0.135, 0.096)0.78 The annual rate of validated infections (Number of Infections/year/subject) was 0.18 in the group treated with GAMUNEX-C and 0.43 in the group treated with GAMIMUNE N, 10% (p=0.023). The annual rates for any infection (validated plus clinically-defined, non-validated infections of any organ system) were 2.88 and 3.38, respectively (p=0.300).
ITP
A double-blind, randomized, parallel group clinical trial with 97 ITP subjects was carried out to test the hypothesis that GAMUNEX-C was at least as effective as GAMIMUNE N, 10% in raising platelet counts from less than or equal to 20 x109/L to more than 50 x109/L within 7 days after treatment with 2 g/kg IGIV. Twenty-four percent of the subjects were less than or equal to 16 years of age.(26)
GAMUNEX-C was demonstrated to be at least as effective as GAMIMUNE N, 10% in the treatment of adults and children with acute or chronic ITP.
Table 14: Platelet Response of Per Protocol Analysis Number of Responders (percent of all subjects) GAMUNEX®-C
(n=39)GAMIMUNE® N, 10%
(n=42)Mean Difference
(90% Confidence Interval)By Day 7 35 (90%) 35 (83%) 0.075
(-0.037, 0.186)By Day 23 35 (90%) 36 (86%) 0.051
(-0.058, 0.160)Sustained for 7 days 29 (74%) 25 (60%) 0.164
(0.003, 0.330)A trial was conducted to evaluate the clinical response to rapid infusion of GAMUNEX-C in patients with ITP. The study involved 28 chronic ITP subjects, wherein the subjects received 1 g/kg GAMUNEX-C on three occasions for treatment of relapses. The infusion rate was randomly assigned to 0.08, 0.11, or 0.14 mL/kg/min (8, 11 or 14 mg/kg/min). Pre-medication with corticosteroids to alleviate infusion-related intolerability was not permitted. Pre-treatment with antihistamines, anti-pyretics and analgesics was permitted. The average dose was approximately 1 g/kg body weight at all three prescribed rates of infusion (0.08, 0.11 and 0.14 mL/kg/min). All patients were administered each of the three planned infusions except seven subjects. Based on 21 patients per treatment group, the a posteriori power to detect twice as many drug-related adverse events between groups was 23%. Of the seven subjects that did not complete the study, five did not require additional treatment, one withdrew because he refused to participate without concomitant medication (prednisone) and one experienced an adverse event (hives); however, this was at the lowest dose rate level (0.08 mL/kg/min).
CIDP
A multi-center, randomized, double-blind, Placebo-controlled trial (The Immune Globulin Intravenous (Human), 10% Caprylate/Chromatography Purified CIDP Efficacy or ICE study) was conducted with GAMUNEX-C.(27) This study included two separately randomized periods to assess whether GAMUNEX-C was more effective than Placebo for the treatment of CIDP (assessed in the Efficacy Period for up to 24 weeks) and whether long-term administration of GAMUNEX-C could maintain long-term benefit (assessed in the 24 week Randomized Withdrawal Period).
In the Efficacy Period, there was a requirement for Rescue (crossover) to the alternate study drug if the subject did not improve and maintain this improvement until the end of the 24 week treatment period. Subjects entering the Rescue phase followed the same dosing and schedule as in the Efficacy period. Any subject who was rescued (crossed over) and did not improve and maintain this improvement was withdrawn from the study.
Subjects who completed 24 weeks treatment in the Efficacy period or Rescue phase and responded to therapy were eligible for entry into a double-blind Randomized Withdrawal Period. Eligible subjects were re-randomized to GAMUNEX-C or Placebo. Any subject who relapsed was withdrawn from the study.
The Efficacy Period and the Rescue treatment started with a loading dose of 2 g/kg body weight of GAMUNEX-C or equal volume of Placebo given over 2-4 consecutive days. All other infusions (including the first infusion of the Randomized Withdrawal Period) were given as maintenance doses of 1 g/kg body weight (or equivalent volume of Placebo) every three weeks.
The Responder rates of the GAMUNEX-C and Placebo treatment groups were measured by the INCAT score. The INCAT (Inflammatory Neuropathy Cause and Treatment) scale is used to assess functional disability of both upper and lower extremities in demyelinating polyneuropathy. The INCAT scale has upper and lower extremity components (maximum of 5 points for upper (arm disability) and maximum of 5 points for lower (leg disability)) that add up to a maximum of 10-points (0 is normal and 10 is severely incapacitated).(28) At the start of the efficacy portion of the study, the INCAT scores were as follows: Upper Extremity mean was 2.2 ± 1.0, and median was 2.0 with a range of 0 to 5; Lower Extremity mean was 1.9 ± 0.9, and median was 2.0 with a range of 1 to 5; Total Overall Score mean was 4.2 ± 1.4, and median was 4.0 with a range of 2 to 9. A Responder was defined as a subject with at least 1-point improvement from baseline in the adjusted INCAT score that was maintained through 24 weeks.
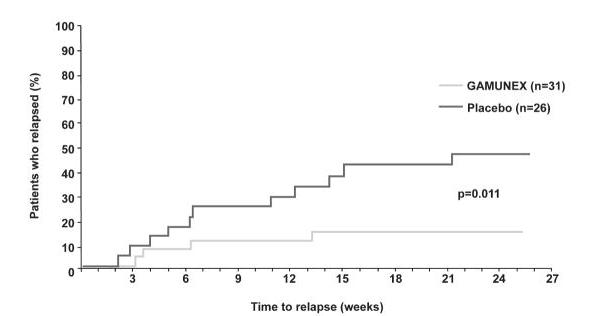
More subjects with CIDP responded to GAMUNEX-C: 28 of 59 subjects (47.5%) responded to GAMUNEX-C compared with 13 of 58 subjects (22.4%) administered Placebo (25% difference; 95% CI 7%-43%; p=0.006). The study included both subjects who were IGIV naïve and subjects who had previous IGIV experience. The outcome was influenced by the group of subjects who experienced prior therapy with IGIV, as shown by the outcomes table, below.
Time to relapse for the subset of 57 subjects who previously responded to GAMUNEX-C was evaluated: 31 were randomly reassigned to continue to receive GAMUNEX-C and 26 subjects were randomly reassigned to Placebo in the Randomized Withdrawal Period. Subjects who continued to receive GAMUNEX-C experienced a longer time to relapse versus subjects treated with Placebo (p=0.011). The probability of relapse was 13% with GAMUNEX-C versus 45% with Placebo (hazard ratio, 0.19; 95% confidence interval, 0.05, 0.70).
Table 15: Outcomes in Intent-to-Treat Population Efficacy Period - * p-value based on Fisher's exact method
Efficacy Period GAMUNEX®-C Placebo p-value* Responder Non-Responder Responder Non-Responder All Subjects 28/59 (47.5%) 31/59 (52.5%) 13/58 (22.4%) 45/58 (77.6%) 0.006 IGIV -
Naïve Subjects
17/39 (43.6%)
22/39 (56.4%)
13/46 (28.3%)
33/46 (71.7%)
0.174IGIV -
Experienced Subjects
11/20 (55.0%)
9/20 (45.0%)
0/12 (0%)
12/12 (100%)
0.002The following table shows outcomes for the Rescue Phase (which are supportive data):
Table 16: Outcomes in Rescue Phase - * p-value based on Fisher's exact method
Rescue Phase GAMUNEX®-C Placebo p-value* Success Failure Success Failure All Subjects 25/45
(55.6%)20/45
(44.4%)6/23
(26.1%)17/23
(73.9%)0.038 IGIV - Naïve Subjects 19/33
(57.6%)14/33
(42.4%)6/18
(33.3%)12/18
(66.7%)0.144 IGIV - Experienced Subjects 6/12
(50%)6/12
(50%)0/5
(0%)5/5
(100%)0.102 The following Kaplan-Meier curves show the outcomes for the Randomized Withdrawal Period:
-
15 REFERENCES
- Buckley RH, Schiff RI. The use of intravenous immune globulin in immunodeficiency diseases. N Engl J Med 1991;325(2):110-7.
- Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 1999;92(1):34-48.
- Pruzanski W, Sussman G, Dorian W, et al. Relationship of the dose of intravenous gammaglobulin to the prevention of infections in adults with common variable immunodeficiency. Inflammation 1996;20(4):353-9.
- Stephan JL, Vlekova V, Le Deist F, et al. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J Pediatr 1993;123(4):564-72.
- Blanchette VS, Kirby MA, Turner C. Role of intravenous immunoglobulin G in autoimmune hematologic disorders. Semin Hematol 1992;29(3 Suppl 2):72-82.
- Lazarus AH, Freedman J, Semple JW. Intravenous immunoglobulin and anti-D in idiopathic thrombocytopenic purpura (ITP): mechanisms of action. Transfus Sci 1998;19(3):289-94.
- Cayco AV, Perazella MA, Hayslett JP. Renal insufficiency after intravenous immune globulin therapy: a report of two cases and an analysis of the literature. J Am Soc Nephrol 1997;8(11):1788-94.
- Pierce LR, Jain N. Risks associated with the use of intravenous immunoglobulin. Trans Med Rev 2003;17:241-51.
- Steinberger BA, Ford SM, Coleman TA. Intravenous immunoglobulin therapy results in post-infusional hyperproteinemia, increased serum viscosity, and pseudohyponatremia. Am J Hematol 2003;73:97-100.
- Dalakas MC. High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 1994;44:223-6.
- Woodruff RK, Grigg AP, Firkin FC, et al. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet 1986;2:217-8.
- Wolberg AS, Kon RH, Monroe DM, et al. Coagulation factor XI is a contaminant in intravenous immunoglobulin preparations. Am J Hematol 2000;65:30-4.
- Copelan EA, Strohm PL, Kennedy MS, et al. Hemolysis following intravenous immune globulin therapy. Transfusion 1986;26:410-2.
- Thomas MJ, Misbah SA, Chapel HM, et al. Hemolysis after high-dose intravenous Ig. Blood 1993;15:3789.
- Wilson JR, Bhoopalam N, Fisher M. Hemolytic anemia associated with intravenous immunoglobulin. Muscle & Nerve 1997;20:1142-5.
- Kessary-Shoham H, Levy Y, Shoenfeld Y, et al. In vivo administration of intravenous immunoglobulin (IVIg) can lead to enhanced erythrocyte sequestration. J Autoimmune 1999;13:129-35.
- Kahwaji J, Barker E, Pepkowitz S, et al. Acute hemolysis after high-dose intravenous immunoglobulin therapy in highly HLA sensitized patients. Clin J Am Soc Nephrol 2009;4:1993-7.
- Daw Z, Padmore R, Neurath D, et al. Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: A case series analysis. Transfusion 2008;48:1598-601.
- Rizk A, Gorson KC, Kenney L, et al. Transfusion-related acute lung injury after the infusion of IVIG. Transfusion 2001;41:264-8.
- Orbach H, Katz U, Sherer Y, et al. Intravenous immunoglobulin: adverse effects and safe administration. Clin Rev Allergy Immunol 2005;29:173-84.
- Tai VM, Mitchell EJ, Lee-Brotherton V, et al. Safety evaluation of intravenous glycine in formulation development. J Pharm Pharmaceut Sci 2000;3:198.
- Traul KA, Driedger A, Ingle D, et al. Review of the toxicologic properties of medium-chain triglycerides. Food Chem Toxicol 2000;38(1):79-98.
- Barnette D, Roth NJ, Hotta J, et al. Pathogen safety profile of a 10% IgG preparation manufactured using a depth filtration-modified process. Biologicals 2012;40:247-53.
- Wasserman RL, Irani A-M, Tracy J, et al. Pharmacokinetics and safety of subcutaneous immune globulin (human), 10% caprylate/chromatography purified in patients with primary immunodeficiency disease. Clinical and Experimental Immunology 2011;161:518-26.
- Roifman CM, Schroeder H, Berger M, et al. Comparison of the efficacy of IGIV-C, 10% (caprylate/chromatography) and IGIV-SD, 10% as replacement therapy in primary immune deficiency. A randomized double-blind trial. Internat Immunopharmacol 2003;3:1325–33.
- Bussell JB, Eldor A, Kelton JG, et al. IGIV-C, a novel intravenous immunoglobulin: evaluation of safety, efficacy, mechanisms of action and impact on quality of life. Thromb Haemost 2004;91:771–8.
- Hughes RAC, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate/chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol 2008;7:136-44.
- Hughes R, Bensa S, Willison H, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol 2001;50(2):195-201.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
GAMUNEX-C is supplied in single-use, tamper evident vials (shrink band) containing the labeled amount of functionally active IgG. The four larger vial size labels incorporate integrated hangers. GAMUNEX-C is not made with natural rubber latex.
GAMUNEX-C is supplied in the following sizes:
NDC Number Size Grams Protein 13533-800-12 10 mL 1 13533-800-15 25 mL 2.5 13533-800-20 50 mL 5 13533-800-71 100 mL 10 13533-800-24 200 mL 20 13533-800-40 400 mL 40 - DO NOT FREEZE
- Keep the vial in the carton to protect from light.
- GAMUNEX-C may be stored for 36 months at 2-8°C (36-46°F) from the date of manufacture, AND product may be stored at temperatures not to exceed 25°C (77°F) for up to 6 months anytime during the 36 month shelf life, after which the product must be immediately used or discarded.
- Do not use after expiration date.
-
17 PATIENT COUNSELING INFORMATION
[see Boxed Warning and Warnings and Precautions]
Instruct patients to immediately report the following signs and symptoms to their healthcare provider:
- Decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath [see Warnings and Precautions (5.2)]
- Symptoms of thrombosis which may include: pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body [see Warnings and Precautions (5.4)]
- Severe headache, neck stiffness, drowsiness, fever, sensitivity to light, painful eye movements, nausea, and vomiting [see Warnings and Precautions (5.5)]
- Increased heart rate, fatigue, yellowing of the skin or eyes, and dark-colored urine [see Warnings and Precautions (5.6)]
- Trouble breathing, chest pain, blue lips or extremities, and fever [see Warnings and Precautions (5.7)]
Inform patients that GAMUNEX-C is made from human plasma and may contain infectious agents that can cause disease. While the risk GAMUNEX-C can transmit an infectious agent has been reduced by screening plasma donors for prior exposure, testing donated plasma, and by inactivating or removing pathogens during manufacturing, patients should report any symptoms that concern them. [see Warnings and Precautions (5.9)]
Inform patients that GAMUNEX-C can interfere with their immune response to live virus vaccines such as measles, mumps and rubella. Inform patients to notify their healthcare professional of this potential interaction when they are receiving vaccinations. [see Drug Interactions (7)]
PI: Self-Administration: Subcutaneous Administration Only
Advise the patient to read the FDA-approved patient labeling (Instructions for Use: Subcutaneous Infusion for Primary Humoral Immunodeficiency).
Provide the patient with instructions on subcutaneous infusion for home treatment, if the physician believes that home administration is appropriate for the patient.
- The type of equipment to be used along with its maintenance,
- proper infusion techniques, selection of appropriate infusion sites (e.g., abdomen, thighs, upper arms, and/or lateral hip),
- maintenance of a treatment diary, and
- measures to be taken in case of adverse reactions in the patient instructions.
Manufactured by:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
3054846/3054847/3056967GAMUNEX®-C
Immune Globulin Injection (Human),
10% Caprylate/Chromatography PurifiedInstructions for Use: Subcutaneous Infusion for
Primary Humoral ImmunodeficiencyInformation for Patients
Please read this information about GAMUNEX-C carefully before using this medicine. This information does not take the place of talking with your healthcare professional, and it does not include all of the important information about GAMUNEX-C. If you have any questions after reading this, contact your healthcare professional.What is the most important information I should know about GAMUNEX-C?
GAMUNEX-C should be infused under your skin (in the subcutaneous tissue). DO NOT inject GAMUNEX-C into a blood vessel or directly into a muscle.What is GAMUNEX-C?
GAMUNEX-C (Găm-yōō-nĕx) is an immunoglobulin used to treat primary immune deficiency (PI). Immunoglobulin is another name for the purified antibodies from human plasma that defend the body against infections such as viruses and bacteria. People with PI lack the healthy antibodies needed to fight off these infections. GAMUNEX-C provides those healthy antibodies and will help lower the number and severity of infections you could get.Who should NOT take GAMUNEX-C?
Do not take GAMUNEX-C if you have known severe allergic reactions or a severe response to Immune Globulin (Human). Tell your doctor if you have had a serious reaction to other medicines that contain immune globulin. Also tell your doctor if you have an immunoglobulin A (IgA) deficiency.How should I take GAMUNEX-C?
You will take GAMUNEX-C through infusions given just below the skin (in the subcutaneous tissue). As directed by your physician, one or more injection sites on your body will be selected. The number and location of the injection sites depends on the amount you need to receive. Typically, adults may use 1 to 4 needles in different locations at one time. You may use up to 8 needles as directed by your doctor. For children, use up to 6 infusion sites simultaneously. For patients of all ages ensure that the infusion sites are at least 2 inches (5 cm) apart. The needles are attached with a tube to the pump. You will need to have infusions once a week.Instructions for administering GAMUNEX-C are at the end of this patient Instructions for Use [see "Steps for Administration"]. Only use GAMUNEX-C by yourself after you have been instructed by your doctor or healthcare professional.
What should I avoid while taking GAMUNEX-C?
Certain types of vaccines (ones containing a live virus) may not work as well for you if you are also receiving immunoglobulin products like GAMUNEX-C. The antibodies in GAMUNEX-C may prevent the vaccine from working. Before you get a vaccine, tell the doctor or nurse that you are taking GAMUNEX-C.Tell your doctor or healthcare professional if you are pregnant or plan to become pregnant, or if you are nursing.
What are possible side effects of GAMUNEX-C?
The most common side effects with GAMUNEX-C when given under the skin (subcutaneously) are:- Redness, swelling, and itching at the injection site
- Fatigue
- Headache
- Pain (including pain in the joints, arms, legs)
- Diarrhea
- Nausea
- Migraine
- Fever
Tell your doctor right away or go to the emergency room if you have hives, trouble breathing, wheezing, dizziness, or fainting. These could be signs of a bad allergic reaction.
Tell your doctor right away if you have any of the following symptoms. They could be signs of a rare, but serious problem.
- Decreased urination, sudden weight gain, fluid retention/swelling in your legs, and/or shortness of breath. They could be signs of a serious kidney problem called renal failure.
- Pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body. These could be signs of a blood clot in your body (thrombosis). Immediately report symptoms of thrombosis.
- Severe headache, stiff neck, fatigue, fever, sensitivity to light, painful eye movements, nausea and vomiting. These could be signs of a type of brain inflammation called aseptic meningitis.
- Increased heart rate, fatigue, yellow skin or eyes, and dark colored urine. These could be signs of a type of blood problem called hemolytic anemia.
- Chest pains, trouble breathing, blue lips or extremities, and fever. These could be signs of a lung problem called TRALI (transfusion-related acute lung injury).
- Fever over 100°F (37.8oC). This could be a sign of an infection.
Tell your doctor about any side effects that concern you. You can ask your doctor to give you the full prescribing information available to healthcare professionals.
Steps for Administration
Infuse GAMUNEX-C only after you have been trained by your doctor or healthcare professional. Below are step-by-step instructions to help you remember how to use GAMUNEX-C. Ask your doctor or healthcare professional about any instructions you do not understand.Before Using GAMUNEX-C
- GAMUNEX-C comes in single-use vials.
- Keep it refrigerated. Do not let it freeze.
- Keep the vial in the carton to protect from light.
- GAMUNEX-C can be stored at room temperature for up to 6 months but you must use it within that time or you must throw it away.
- Do not shake the vials.
- Prior to use, allow the solution to come to room temperature (68-77°F or 20-25°C). This can take 60 minutes or longer.
- Do not use the vial if:
- the solution is cloudy, discolored or contains particles. The solution should be clear to opalescent, and colorless to pale yellow.
- the protective cap or plastic shrink band around the cap is missing, or there is any evidence of tampering. Tell your healthcare provider immediately.
- the expiration date has passed.
- Sanitize your infusion set-up area by preparing a clean, flat, non-porous surface such as a kitchen counter. Avoid using porous surfaces such as wood. Clean the surface with an alcohol wipe using a circular motion from the center outward.
Step 1:
Wash and dry your hands thoroughly before administering GAMUNEX-C- Your healthcare provider may recommend that you use antibacterial soap or that you wear gloves.
Step 2:
Remove the protective cap and sanitize the stopper- Remove the protective cap from the vial to expose the central portion of the stopper.
- Wipe the stopper with alcohol and allow to dry.
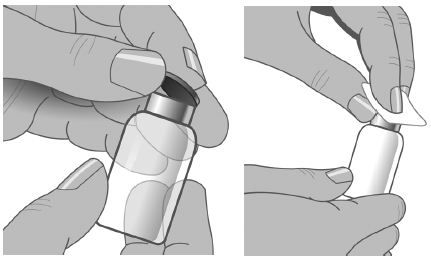
Step 3:
Use aseptic technique when preparing and administering GAMUNEX-C- Do not allow your fingers or other objects to touch the inner stem of the plunger, the syringe tip, or other areas that will come in contact with your GAMUNEX-C solution. This is called aseptic technique and is designed to prevent transmission of germs.
- Using aseptic technique, attach each needle to the syringe tip.
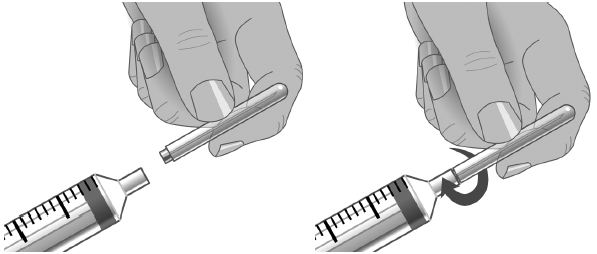
Step 4:
Prepare the syringe and draw GAMUNEX-C solution into syringe- Remove cap from needle.
- Pull the syringe plunger back to the level matching the amount of GAMUNEX-C to be withdrawn from the vial.
- Place the GAMUNEX-C vial on a clean flat surface and insert the needle into the center of the vial stopper.
- Inject air into the vial. The amount of air should match the amount of GAMUNEX-C to be withdrawn.
- Turn the vial upside down and withdraw the correct amount of GAMUNEX-C. If multiple vials are required to achieve the correct dose, repeat Step 4.
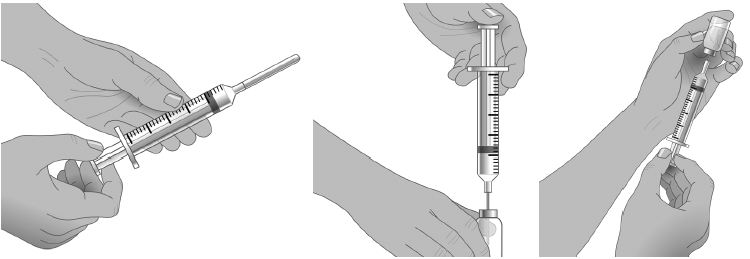
Step 5:
Fill the pump reservoir and prepare the infusion pump- Follow the pump manufacturer’s instructions for filling the pump reservoir and preparing the infusion pump, administration tubing and Y-site connection tubing, if needed.
- Be sure to prime the administration tubing to ensure that no air is left in the tubing or needle by filling the tubing/needle with GAMUNEX-C. To prime, hold the syringe in one hand and the administration tubing’s capped needle in the other. Gently squeeze on the plunger until you see a drop of GAMUNEX-C exit from the needle.
Example Equipment
Step 6:
Select the number and location of infusion sites- Select one or more infusion sites as directed by your healthcare provider.
- The number and location of injection sites depends on the volume of the total dose.
Step 7:
Prepare the infusion site- Cleanse the infusion site(s) with antiseptic solution using a circular motion working from the center of the site and moving to the outside.
- Sites should be clean, dry, and at least 2 inches apart.
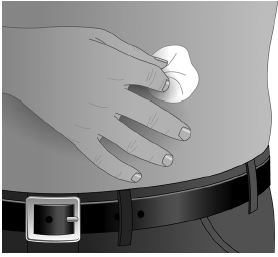
Step 8:
Insert the needle- Grasp the skin between two fingers and insert the needle into the subcutaneous tissue.
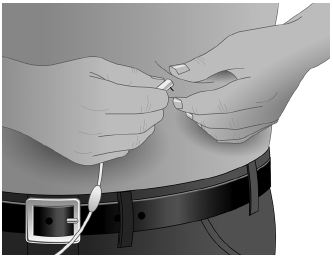
Step 9:
Do not inject GAMUNEX-C into a blood vessel- After inserting each needle into tissue (and before your infusion), make sure that a blood vessel has not been accidentally entered. To do this, attach a sterile syringe to the end of the primed administration tubing. Pull back on the syringe plunger and watch for any blood flowing back into administration tubing.
- If you see any blood, remove and discard the needle and administration tubing.
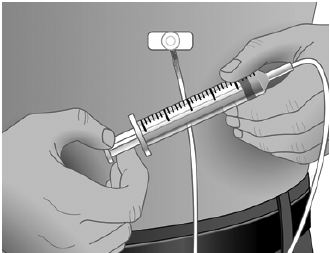
- Repeat priming and needle insertion steps using a new needle, administration tubing and a new infusion site.
- Secure the needle in place by applying sterile gauze or transparent dressing over the site.
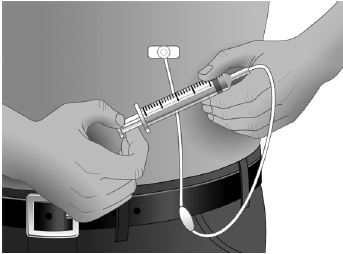
Step 10:
Repeat for other sites, as needed- If using multiple, simultaneous infusion sites, use Y-site connection tubing and secure to the administration tubing.
Step 11:
Infuse GAMUNEX-C following the pump manufacturer’s instructions for the infusion pump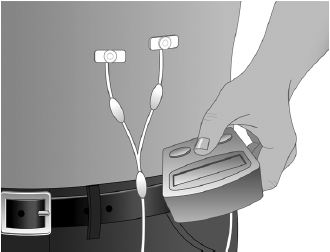
Step 12:
After infusion, turn off pump and dispose of used supplies- Follow manufacturer’s instructions to turn off pump.
- Undo and discard any dressing or tape.
- Gently remove the inserted needle(s) or catheter(s).
- Discard any unused solution in an appropriate waste container as instructed.
-
Discard any used administration equipment in an appropriate waste container.
- Store your supplies in a safe place.
- Follow manufacturer’s instructions to care for the infusion pump.
Step 13:
Record each infusion- Remove the peel-off label with the product lot number from the GAMUNEX-C vial and use this to complete the patient record.
- Remember to bring your journal with you when you visit your physician or healthcare provider.
Be sure to tell your doctor about any problems you have doing your infusions. Your doctor may ask to see your journal, so be sure to take it with you each time you visit the doctor’s office.
Call your doctor for medical advice about side effects. You can also report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured by:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871Revised 1/2020
3054846/3054847/3056967
-
PACKAGE LABEL
Immune Globulin Injection (Human), 10%
gamunex®-c
Caprylate/Chromatography Purified
Solution for Infusion
NDC 13533-800-71
10 Grams Protein in 100 ml
GRIFOLS
GRIFOLS
10 Grams Protein in 100 ml
Contents: One vial of Gamunex-C
No preservative
Sterile, Non-pyrogenic
Keep the vial in the carton to protect from light.
Gamunex-C may be stored for 36 months at 2–8°C (36–46°F), AND product may be stored at temperatures not to exceed 25°C (77°F) for up to 6 months anytime during the 36 month shelf life, after which the product must be immediately used or discarded.
Date removed from refrigeration:
The patient and physician should discuss the risks and benefits of this product.
FOR INTRAVENOUS OR SUBCUTANEOUS ADMINISTRATION.
Refer to package insert for dosage and administration and compatibility with other solutions.
Single Dose Vial
Do not use if turbid.
Discard unused portion. Do not store after entry into vial.
Contains no preservative
Do not freeze.
Each milliliter (mL) contains approximately 100 mg of protein, not less than 98% of which is immunoglobulin, and approximately 15 mg glycine.
If the shrink band is absent or shows any sign of tampering, do not use the product and notify Grifols Therapeutics LLC immediately.
Not Returnable for Credit or Exchange
Rx only
Grifols Therapeutics LLC
Research Triangle Park,
NC 27709 USA
U.S. License No. 1871
Immune Globulin Injection (Human), 10%
gamunex®-c
Caprylate/Chromatography Purified
Solution for Infusion
10 Grams Protein in 100 ml
GTIN XXXXXXXXXXXXXX
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXXXXXXXX
Carton: 3056389
NDC: 13533-800-72
Immune Globulin Injection (Human), 10%
gamunex®-c
Caprylate/Chromatography Purified
Solution for Infusion
10 Grams Protein in 100 ml
GRIFOLS
Grifols Therapeutics LLC
Research Triangle Park,
NC 27709 USA
U.S. License No. 1871
The patient and physician should discuss the risks and benefits of this product.
FOR INTRAVENOUS OR SUBCUTANEOUS ADMINISTRATION.
Refer to package insert for dosage and administration and compatibility with other solutions.
Gamunex-C may be stored for 36 months at 2–8°C (36–46°F), AND product may be stored at temperatures not to exceed 25°C (77°F) for up to 6 months anytime during the 36 month shelf life, after which the product must be immediately used or discarded.
Single Dose Vial
Do not use if turbid.
Rx only
Gamunex-C
10 Grams Protein in 100 ml
Lot
3053094
Lot
Exp.

-
INGREDIENTS AND APPEARANCE
GAMUNEX-C
immune globulin (human) injectionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 13533-800 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Human Immunoglobulin G (UNII: 66Y330CJHS) (Human Immunoglobulin G - UNII:66Y330CJHS) Human Immunoglobulin G 10 g in 100 mL Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Product Characteristics Color WHITE (Clear to opalescent liquid, colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13533-800-12 1 in 1 CARTON 1 NDC: 13533-800-13 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC: 13533-800-15 1 in 1 CARTON 2 NDC: 13533-800-16 25 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 3 NDC: 13533-800-20 1 in 1 CARTON 3 NDC: 13533-800-21 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 4 NDC: 13533-800-71 1 in 1 CARTON 4 NDC: 13533-800-72 100 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 5 NDC: 13533-800-24 1 in 1 CARTON 5 NDC: 13533-800-25 200 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 6 NDC: 13533-800-40 1 in 1 CARTON 6 NDC: 13533-800-41 400 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125046 10/13/2010 Labeler - GRIFOLS USA, LLC (048987452) Establishment Name Address ID/FEI Business Operations GRIFOLS THERAPEUTICS LLC 611019113 manufacture(13533-800) Establishment Name Address ID/FEI Business Operations GRIFOLS BIOLOGICALS LLC 092694538 manufacture(13533-800) Establishment Name Address ID/FEI Business Operations GRIFOLS BIOLOGICALS LLC 121076871 manufacture(13533-800)
Trademark Results [Gamunex-C]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GAMUNEX-C 85011751 3979311 Dead/Cancelled |
GRIFOLS THERAPEUTICS LLC 2010-04-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.