PALONOSETRON injection, solution
Palonosetron by
Drug Labeling and Warnings
Palonosetron by is a Prescription medication manufactured, distributed, or labeled by Fresenius Kabi USA, LLC, Fresenius Kabi Austria. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PALONOSETRON HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for PALONOSETRON HYDROCHLORIDE INJECTION.
PALONOSETRON HYDROCHLORIDE injection, for intravenous use
Initial U.S. Approval: 2003INDICATIONS AND USAGE
Palonosetron Hydrochloride Injection is a serotonin-3 (5-HT3) receptor antagonist indicated in adults for:
Moderately emetogenic cancer chemotherapy -- prevention of acute and delayed nausea and vomiting associated with initial and repeat courses (1.1)
Highly emetogenic cancer chemotherapy -- prevention of acute nausea and vomiting associated with initial and repeat courses (1.1)
Palonosetron Hydrochloride Injection is indicated in pediatric patients aged 1 month to less than 17 years for:
Prevention of acute nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including highly emetogenic cancer chemotherapy (1.2)DOSAGE AND ADMINISTRATION
Chemotherapy-Induced Nausea and Vomiting (2.1)
Age
Dose*
Infusion Time
Adults
0.25 mg x 1
Infuse over 30 seconds beginning approx. 30 min before the start of chemo
Pediatrics (1 month to less than 17 years)
20 micrograms per kilogram (max 1.5 mg) x 1
Infuse over 15 minutes beginning approx. 30 min before the start of chemo
*Note different dosing units in pediatrics
Instructions for Intravenous Administration
For a dose of 0.25 mg, use the entire contents (5 mL) of the prefilled syringe.
Do not use the prefilled syringe to administer a dose of less than 0.25 mg (5
mL). (2.2)DOSAGE FORMS AND STRENGTHS
0.25 mg (free base) per 5 mL (concentration: 0.05 mg per mL, 50 mcg per mL) single-dose prefilled syringe (3)
CONTRAINDICATIONS
Palonosetron Hydrochloride Injection is contraindicated in patients known to have hypersensitivity to the drug or any of its components (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity reactions, including anaphylaxis, have been reported with or without known hypersensitivity to other selective 5-HT3
receptor antagonists (5.1)
Serotonin syndrome has been reported with 5-HT3 receptor antagonists alone but particularly with concomitant use of serotonergic drugs (5.2)
ADVERSE REACTIONS
The most common adverse reactions in chemotherapy-induced nausea and vomiting in adults (incidence ≥ 5%) are headache and constipation (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
The potential for clinically significant drug interactions with palonosetron appears to be low (7)
USE IN SPECIFIC POPULATIONS
Chemotherapy-Induced Nausea and Vomiting
Pediatric use: Safety and effectiveness in neonates (less than 1 month of age) have not been established (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Chemotherapy-Induced Nausea and Vomiting in Adults
1.2 Chemotherapy-Induced Nausea and Vomiting in Pediatric Patients Aged 1 month to Less than 17 Years
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Instructions for Intravenous Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Serotonin Syndrome
6 ADVERSE REACTIONS
6.1 Chemotherapy-Induced Nausea and Vomiting
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Race
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Chemotherapy-Induced Nausea and Vomiting in Adults
14.2 Chemotherapy-Induced Nausea and Vomiting in Pediatrics
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Chemotherapy-Induced Nausea and Vomiting in Adults
Palonosetron Hydrochloride Injection is indicated for:
- Moderately emetogenic cancer chemotherapy -- prevention of acute and delayed nausea and vomiting associated with initial and repeat courses
- Highly emetogenic cancer chemotherapy -- prevention of acute nausea and vomiting associated with initial and repeat courses
1.2 Chemotherapy-Induced Nausea and Vomiting in Pediatric Patients Aged 1 month to Less than 17 Years
Palonosetron Hydrochloride Injection is indicated for prevention of acute nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including highly emetogenic cancer chemotherapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
Chemotherapy-Induced Nausea and Vomiting
Age Dose* Infusion Time Adults 0.25 mg x 1 Infuse over 30 seconds
beginning approx. 30
min before the start of
chemoPediatrics (1 month to
less than 17 years)20 micrograms
per kilogram
(max 1.5 mg) x 1Infuse over 15 minutes
beginning approx. 30
min before the start of
chemo
* Note different dosing units in pediatrics
2.2 Instructions for Intravenous Administration
Palonosetron Hydrochloride Injection is supplied ready for intravenous administration at a concentration of 0.05 mg/mL (50 mcg/mL). Palonosetron Hydrochloride Injection should not be mixed with other drugs. The infusion line should be flushed with normal saline before and after administration of Palonosetron Hydrochloride Injection. Parenteral drug products should be inspected visually for particulate matter and discoloration before administration, whenever solution and container permit. Expel air from syringe prior to administration. For a dose of 0.25 mg, use the entire contents (5 mL) of the prefilled syringe. Do not use the prefilled syringe to administer a dose less than 0.25 mg (5 mL).
Use aseptic technique while handling the syringe.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Palonosetron Hydrochloride Injection is contraindicated in patients known to have hypersensitivity to the drug or any of its components [see Adverse Reactions (6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Hypersensitivity reactions, including anaphylaxis, have been reported with or without known hypersensitivity to other 5-HT3 receptor antagonists.
5.2 Serotonin Syndrome
The development of serotonin syndrome has been reported with 5-HT3 receptor antagonists. Most reports have been associated with concomitant use of serotonergic drugs (e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors, mirtazapine, fentanyl, lithium, tramadol, and intravenous methylene blue). Some of the reported cases were fatal. Serotonin syndrome occurring with overdose of another 5-HT3 receptor antagonist alone has also been reported. The majority of reports of serotonin syndrome related to 5-HT3 receptor antagonist use occurred in a post-anesthesia care unit or an infusion center.
Symptoms associated with serotonin syndrome may include the following combination of signs and symptoms: mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, with or without gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome, especially with concomitant use of Palonosetron Hydrochloride Injection and other serotonergic drugs. If symptoms of serotonin syndrome occur, discontinue Palonosetron Hydrochloride Injection and initiate supportive treatment. Patients should be informed of the increased risk of serotonin syndrome, especially if Palonosetron Hydrochloride Injection is used concomitantly with other serotonergic drugs [see Drug Interactions (7), Patient Counseling Information (17)].
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Chemotherapy-Induced Nausea and Vomiting
Adults
In clinical trials for the prevention of nausea and vomiting induced by moderately or highly emetogenic chemotherapy, 1,374 adult patients received palonosetron. Adverse reactions were similar in frequency and severity with Palonosetron Hydrochloride Injection and ondansetron or dolasetron. Following is a listing of all adverse reactions reported by ≥ 2% of patients in these trials (Table 1).
Table 1: Adverse Reactions from Chemotherapy-Induced Nausea and
Vomiting Studies ≥ 2% in any Treatment Group
Event
Palonosetron
Hydrochloride
Injection
0.25 mg
(N=633)
Ondansetron
32 mg I.V.
(N=410)
Dolasetron 100 mg I.V.
(N=194)
Headache
60 (9%)
34 (8%)
32 (16%)
Constipation
29 (5%)
8 (2%)
12 (6%)
Diarrhea
8 (1%)
7 (2%)
4 (2%)
Dizziness
8 (1%)
9 (2%)
4 (2%)
Fatigue
3 (< 1%)
4 (1%)
4 (2%)
Abdominal Pain
1 (< 1%)
2 (< 1%)
3 (2%)
Insomnia
1 (< 1%)
3 (1%)
3 (2%)
In other studies, 2 subjects experienced severe constipation following a single palonosetron dose of approximately 0.75 mg, three times the recommended dose. One patient received a 10 mcg/kg oral dose in a postoperative nausea and vomiting study and one healthy subject received a 0.75 mg I.V. dose in a pharmacokinetic study.
In clinical trials, the following infrequently reported adverse reactions, assessed by investigators as treatment-related or causality unknown, occurred following administration of Palonosetron Hydrochloride Injection to adult patients receiving concomitant cancer chemotherapy:
Cardiovascular: 1%: non-sustained tachycardia, bradycardia, hypotension, < 1%: hypertension, myocardial ischemia, extrasystoles, sinus tachycardia, sinus arrhythmia, supraventricular extrasystoles and QT prolongation. In many cases, the relationship to Palonosetron Hydrochloride Injection was unclear.
Dermatological: < 1%: allergic dermatitis, rash.
Hearing and Vision: < 1%: motion sickness, tinnitus, eye irritation and amblyopia.
Gastrointestinal System: 1%: diarrhea, < 1%: dyspepsia, abdominal pain, dry mouth, hiccups and flatulence.
General: 1%: weakness, < 1%: fatigue, fever, hot flash, flu-like syndrome.
Liver: < 1%: transient, asymptomatic increases in AST and/or ALT and bilirubin. These changes occurred predominantly in patients receiving highly emetogenic chemotherapy.
Metabolic: 1%: hyperkalemia, < 1%: electrolyte fluctuations, hyperglycemia, metabolic acidosis, glycosuria, appetite decrease, anorexia.
Musculoskeletal: < 1%: arthralgia.
Nervous System: 1%: dizziness, < 1%: somnolence, insomnia, hypersomnia, paresthesia.
Psychiatric: 1%: anxiety, < 1%: euphoric mood.
Urinary System: < 1%: urinary retention.
Vascular: < 1%: vein discoloration, vein distention.
Pediatrics
In a pediatric clinical trial for the prevention of chemotherapy-induced nausea and vomiting 163 cancer patients received a single 20 mcg/kg (maximum 1.5 mg) intravenous infusion of palonosetron 30 minutes before beginning the first cycle of emetogenic chemotherapy. Patients had a mean age of 8.4 years (range 2 months to 16.9 years) and were 46% male; and 93% white.
The following adverse reactions were reported for palonosetron:
Nervous System: < 1%: headache, dizziness, dyskinesia.
General: < 1%: infusion site pain.
Dermatological: < 1%: allergic dermatitis, skin disorder.
In the trial, adverse reactions were evaluated in pediatric patients receiving palonosetron for up to 4 chemotherapy cycles.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Palonosetron Hydrochloride Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Very rare cases (< 1/10,000) of hypersensitivity reactions including anaphylaxis and anaphylactic shock and injection site reactions (burning, induration, discomfort and pain) were reported from postmarketing experience of Palonosetron Hydrochloride Injection 0.25 mg in the prevention of chemotherapy-induced nausea and vomiting.
-
7 DRUG INTERACTIONS
Palonosetron is eliminated from the body through both renal excretion and metabolic pathways with the latter mediated via multiple CYP enzymes. Further in vitro studies indicated that palonosetron is not an inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1 and CYP3A4/5 (CYP2C19 was not investigated) nor does it induce the activity of CYP1A2, CYP2D6, or CYP3A4/5. Therefore, the potential for clinically significant drug interactions with palonosetron appears to be low.
Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular symptoms) has been described following the concomitant use of 5-HT3 receptor antagonists and other serotonergic drugs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs) [see Warnings and Precautions (5.2)].
Coadministration of 0.25 mg I.V. palonosetron and 20 mg I.V. dexamethasone in healthy subjects revealed no pharmacokinetic drug-interactions between palonosetron and dexamethasone.
In an interaction study in healthy subjects where palonosetron 0.25 mg (I.V. bolus) was administered on day 1 and oral aprepitant for 3 days (125 mg/80 mg/80 mg), the pharmacokinetics of palonosetron were not significantly altered (AUC: no change, Cmax: 15% increase).
A study in healthy volunteers involving single-dose I.V. palonosetron (0.75 mg) and steady state oral metoclopramide (10 mg four times daily) demonstrated no significant pharmacokinetic interaction.
In controlled clinical trials, Palonosetron Hydrochloride Injection has been safely administered with corticosteroids, analgesics, antiemetics/antinauseants, antispasmodics and anticholinergic agents.
Palonosetron did not inhibit the antitumor activity of the five chemotherapeutic agents tested (cisplatin, cyclophosphamide, cytarabine, doxorubicin and mitomycin C) in murine tumor models.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Risk Summary
Adequate and well controlled studies with Palonosetron Hydrochloride Injection have not been conducted in pregnant women. In animal reproduction studies, no effects on embryo-fetal development were observed with the administration of oral palonosetron during the period of organogenesis at doses up to 1,894 and 3,789 times the recommended human intravenous dose in rats and rabbits, respectively. Because animal reproduction studies are not always predictive of human response, Palonosetron Hydrochloride Injection should be used during pregnancy only if clearly needed.
Animal Data
In animal studies, no effects on embryo-fetal development were observed in pregnant rats given oral palonosetron at doses up to 60 mg/kg/day (1,894 times the recommended human intravenous dose based on body surface area) or pregnant rabbits given oral doses up to 60 mg/kg/day (3,789 times the recommended human intravenous dose based on body surface area) during the period of organogenesis.
8.3 Nursing Mothers
It is not known whether Palonosetron Hydrochloride Injection is present in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants and the potential for tumorigenicity shown for palonosetron in the rat carcinogenicity study [see Nonclinical Toxicology (13.1)], a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Chemotherapy-Induced Nausea and Vomiting
Safety and effectiveness of Palonosetron Hydrochloride Injection have been established in pediatric patients aged 1 month to less than 17 years for the prevention of acute nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including highly emetogenic cancer chemotherapy. Use is supported by a clinical trial where 165 pediatric patients aged 2 months to < 17 years were randomized to receive a single dose of palonosetron 20 mcg/kg (maximum 1.5 mg) administered as an intravenous infusion 30 minutes prior to the start of emetogenic chemotherapy [see Clinical Studies (14.2)]. While this study demonstrated that pediatric patients require a higher palonosetron dose than adults to prevent chemotherapy-induced nausea and vomiting, the safety profile is consistent with the established profile in adults [see Adverse Reactions (6.1)].
Safety and effectiveness of Palonosetron Hydrochloride Injection in neonates (less than 1 month of age) have not been established.
8.5 Geriatric Use
Population pharmacokinetics analysis did not reveal any differences in palonosetron pharmacokinetics between cancer patients ≥ 65 years of age and younger patients (18 to 64 years). Of the 1,374 adult cancer patients in clinical studies of palonosetron, 316 (23%) were ≥ 65 years old, while 71 (5%) were ≥ 75 years old. No overall differences in safety or effectiveness were observed between these subjects and the younger subjects, but greater sensitivity in some older individuals cannot be ruled out. No dose adjustment or special monitoring are required for geriatric patients.
No differences in efficacy were observed in geriatric patients for the CINV indication. Palonosetron Hydrochloride Injection efficacy in geriatric patients has not been adequately evaluated.
8.6 Renal Impairment
Mild to moderate renal impairment does not significantly affect palonosetron pharmacokinetic parameters. Total systemic exposure increased by approximately 28% in severe renal impairment relative to healthy subjects. Dosage adjustment is not necessary in patients with any degree of renal impairment.
8.7 Hepatic Impairment
Hepatic impairment does not significantly affect total body clearance of palonosetron compared to the healthy subjects. Dosage adjustment is not necessary in patients with any degree of hepatic impairment.
8.8 Race
Intravenous palonosetron pharmacokinetics was characterized in twenty-four healthy Japanese subjects over the dose range of 3 to 90 mcg/kg. Total body clearance was 25% higher in Japanese subjects compared to Whites, however, no dose adjustment is required. The pharmacokinetics of palonosetron in Blacks has not been adequately characterized.
-
10 OVERDOSAGE
There is no known antidote to Palonosetron Hydrochloride Injection. Overdose should be managed with supportive care.
Fifty adult cancer patients were administered palonosetron at a dose of 90 mcg/kg (equivalent to 6 mg fixed dose) as part of a dose ranging study. This is approximately 25 times the recommended dose of 0.25 mg. This dose group had a similar incidence of adverse events compared to the other dose groups and no dose response effects were observed.
Dialysis studies have not been performed, however, due to the large volume of distribution, dialysis is unlikely to be an effective treatment for palonosetron overdose. A single intravenous dose of palonosetron at 30 mg/kg (947 and 474 times the human dose for rats and mice, respectively, based on body surface area) was lethal to rats and mice. The major signs of toxicity were convulsions, gasping, pallor, cyanosis and collapse.
-
11 DESCRIPTION
Palonosetron hydrochloride is an antiemetic and antinauseant agent. It is a serotonin-3 (5-HT3) receptor antagonist with a strong binding affinity for this receptor. Chemically, palonosetron hydrochloride is: (3aS)-2-[(S)-1-Azabicyclo [2.2.2]oct-3-yl]-2,3,3a,4,5,6-hexahydro-1-oxo-1Hbenz[de]isoquinoline hydrochloride. Palonosetron hydrochloride exists as a single isomer and has the following structural formula:
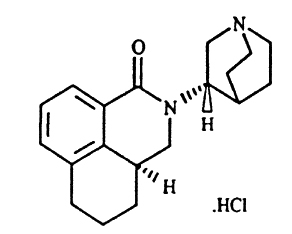
C19H24N2OHCl M.W. 332.87
Palonosetron hydrochloride is a white to off-white crystalline powder. It is freely soluble in water, soluble in propylene glycol, and slightly soluble in ethanol and 2-propanol.
Palonosetron Hydrochloride Injection is a sterile, clear, colorless, non pyrogenic, isotonic, buffered solution for intravenous administration. Palonosetron Hydrochloride Injection is available as a 5 mL single-dose prefilled syringe. Each 5 mL syringe contains 0.25 mg palonosetron base as 0.28 mg palonosetron hydrochloride, 202.4 mg mannitol, 2.5 mg edetate disodium dihydrate, 18 mg trisodium citrate dihydrate, and 7 mg citric acid anhydrous in water for intravenous administration.
The pH of the solution in the 5 mL syringe is 4.5 to 5.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Palonosetron is a 5-HT3 receptor antagonist with a strong binding affinity for this receptor and little or no affinity for other receptors.
Cancer chemotherapy may be associated with a high incidence of nausea and vomiting, particularly when certain agents, such as cisplatin, are used. 5-HT3 receptors are located on the nerve terminals of the vagus in the periphery and centrally in the chemoreceptor trigger zone of the area postrema. It is thought that chemotherapeutic agents produce nausea and vomiting by releasing serotonin from the enterochromaffin cells of the small intestine and that the released serotonin then activates 5-HT3 receptors located on vagal afferents to initiate the vomiting reflex.
12.2 Pharmacodynamics
The effect of palonosetron on blood pressure, heart rate, and ECG parameters including QTc were comparable to ondansetron and dolasetron in CINV clinical trials. In non-clinical studies palonosetron possesses the ability to block ion channels involved in ventricular de- and re-polarization and to prolong action potential duration.
The effect of palonosetron on QTc interval was evaluated in a double blind, randomized, parallel, placebo and positive (moxifloxacin) controlled trial in adult men and women. The objective was to evaluate the ECG effects of I.V. administered palonosetron at single doses of 0.25, 0.75 or 2.25 mg in 221 healthy subjects. The study demonstrated no significant effect on any ECG interval including QTc duration (cardiac repolarization) at doses up to 2.25 mg.
12.3 Pharmacokinetics
After intravenous dosing of palonosetron in healthy subjects and cancer patients, an initial decline in plasma concentrations is followed by a slow elimination from the body. Mean maximum plasma concentration (Cmax) and area under the concentration-time curve (AUC0-∞) are generally dose-proportional over the dose range of 0.3 to 90 mcg/kg in healthy subjects and in cancer patients. Following single I.V. dose of palonosetron at 3 mcg/kg (or 0.21 mg/70 kg) to six cancer patients, mean (± SD) maximum plasma concentration was estimated to be 5,630 ± 5,480 ng/L and mean AUC was 35.8 ± 20.9 hmcg/L.
Following I.V. administration of palonosetron 0.25 mg once every other day for 3 doses in 11 cancer patients, the mean increase in plasma palonosetron concentration from Day 1 to Day 5 was 42 ± 34%. Following I.V. administration of palonosetron 0.25 mg once daily for 3 days in 12 healthy subjects, the mean (± SD) increase in plasma palonosetron concentration from Day 1 to Day 3 was 110 ± 45%.
Distribution
Palonosetron has a volume of distribution of approximately 8.3 ± 2.5 L/kg. Approximately 62% of palonosetron is bound to plasma proteins.
Metabolism
Palonosetron is eliminated by multiple routes with approximately 50% metabolized to form two primary metabolites: N-oxide-palonosetron and 6-S-hydroxy-palonosetron. These metabolites each have less than 1% of the 5-HT3 receptor antagonist activity of palonosetron. In vitro metabolism studies have suggested that CYP2D6 and to a lesser extent, CYP3A4 and CYP1A2 are involved in the metabolism of palonosetron. However, clinical pharmacokinetic parameters are not significantly different between poor and extensive metabolizers of CYP2D6 substrates.
Elimination
After a single intravenous dose of 10 mcg/kg [14C]-palonosetron, approximately 80% of the dose was recovered within 144 hours in the urine with palonosetron representing approximately 40% of the administered dose. In healthy subjects, the total body clearance of palonosetron was 0.160 ± 0.035 L/h/kg and renal clearance was 0.067 ± 0.018 L/h/kg. Mean terminal elimination half-life is approximately 40 hours.
Specific Populations
Pediatric Patients
Single-dose I.V. Palonosetron Hydrochloride Injection pharmacokinetic data was obtained from a subset of pediatric cancer patients that received 10 mcg/kg or 20 mcg/kg. When the dose was increased from 10 mcg/kg to 20 mcg/kg a dose-proportional increase in mean AUC was observed. Following single dose intravenous infusion of Palonosetron Hydrochloride Injection 20 mcg/kg, peak plasma concentrations (CT) reported at the end of the 15 minute infusion were highly variable in all age groups and tended to be lower in patients < 6 years than in older patients. Median half-life was 29.5 hours in overall age groups and ranged from about 20 to 30 hours across age groups after administration of 20 mcg/kg.
The total body clearance (L/h/kg) in patients 12 to 17 years old was similar to that in healthy adults. There are no apparent differences in volume of distribution when expressed as L/kg.
Table 3: Pharmacokinetics Parameters in Pediatric Cancer Patients following intravenous infusion of Palonosetron Hydrochloride Injection at 20 mcg/kg over 15 min
PK Parameter a
Pediatric Age Group
< 2 y
2 to < 6 y
6 to < 12 y
12 to < 17 y
N=12
N=42
N=38
N=44
CTb, ng/L
9,025 (197)
9,414 (252)
16,275 (203)
11,831 (176)
N=5
N=7
N=10
AUC0-∞, hmcg/L
103.5 (40.4)
98.7 (47.7)
124.5 (19.1)
N=6
N=14
N=13
N=19
Clearance c, L/h/kg
0.31 (34.7)
0.23 (51.3)
0.19 (46.8)
0.16 (27.8)
Vssc, L/kg
6.08 (36.5)
5.29 (57.8)
6.26 (40.0)
6.20 (29.0)
a Geometric Mean (CV) except for t1/2 which is median values.
b CT is the plasma palonosetron concentration at the end of the 15 minute infusion.
c Clearance and Vss calculated from 10 and 20 mcg/kg and are weight adjusted.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104-week carcinogenicity study in CD-1 mice, animals were treated with oral doses of palonosetron at 10, 30 and 60 mg/kg/day. Treatment with palonosetron was not tumorigenic. The highest tested dose produced a systemic exposure to palonosetron (Plasma AUC) of about 150 to 289 times the human exposure (AUC= 29.8 hmcg/L) at the recommended intravenous dose of 0.25 mg. In a 104-week carcinogenicity study in Sprague-Dawley rats, male and female rats were treated with oral doses of 15, 30 and 60 mg/kg/day and 15, 45 and 90 mg/kg/day, respectively. The highest doses produced a systemic exposure to palonosetron (Plasma AUC) of 137 and 308 times the human exposure at the recommended dose. Treatment with palonosetron produced increased incidences of adrenal benign pheochromocytoma and combined benign and malignant pheochromocytoma, increased incidences of pancreatic Islet cell adenoma and combined adenoma and carcinoma and pituitary adenoma in male rats. In female rats, it produced hepatocellular adenoma and carcinoma and increased the incidences of thyroid C-cell adenoma and combined adenoma and carcinoma.
Palonosetron was not genotoxic in the Ames test, the Chinese hamster ovarian cell (CHO/HGPRT) forward mutation test, the ex vivo hepatocyte unscheduled DNA synthesis (UDS) test or the mouse micronucleus test. It was, however, positive for clastogenic effects in the Chinese hamster ovarian (CHO) cell chromosomal aberration test.
Palonosetron at oral doses up to 60 mg/kg/day (about 1,894 times the recommended human intravenous dose based on body surface area) was found to have no effect on fertility and reproductive performance of male and female rats.
-
14 CLINICAL STUDIES
14.1 Chemotherapy-Induced Nausea and Vomiting in Adults
Efficacy of single-dose palonosetron injection in preventing acute and delayed nausea and vomiting induced by both moderately and highly emetogenic chemotherapy was studied in three Phase 3 trials and one Phase 2 trial. In these double-blind studies, complete response rates (no emetic episodes and no rescue medication) and other efficacy parameters were assessed through at least 120 hours after administration of chemotherapy. The safety and efficacy of palonosetron in repeated courses of chemotherapy was also assessed.
Moderately Emetogenic Chemotherapy
Two Phase 3, double-blind trials involving 1,132 patients compared single-dose I.V. Palonosetron Hydrochloride Injection with either single-dose I.V. ondansetron (study 1) or dolasetron (study 2) given 30 minutes prior to moderately emetogenic chemotherapy including carboplatin, cisplatin ≤ 50 mg/m², cyclophosphamide < 1,500 mg/m², doxorubicin > 25 mg/m², epirubicin, irinotecan, and methotrexate > 250 mg/m². Concomitant corticosteroids were not administered prophylactically in study 1 and were only used by 4 to 6% of patients in study 2. The majority of patients in these studies were women (77%), White (65%) and naïve to previous chemotherapy (54%). The mean age was 55 years.
Highly Emetogenic Chemotherapy
A Phase 2, double-blind, dose-ranging study evaluated the efficacy of single-dose I.V. palonosetron from 0.3 to 90 mcg/kg (equivalent to < 0.1 mg to 6 mg fixed dose) in 161 chemotherapy-naïve adult cancer patients receiving highly-emetogenic chemotherapy (either cisplatin ≥ 70 mg/m² or cyclophosphamide > 1,100 mg/m²). Concomitant corticosteroids were not administered prophylactically. Analysis of data from this trial indicates that 0.25 mg is the lowest effective dose in preventing acute nausea and vomiting induced by highly emetogenic chemotherapy.
A Phase 3, double-blind trial involving 667 patients compared single-dose I.V. Palonosetron Hydrochloride Injection with single-dose I.V. ondansetron (study 3) given 30 minutes prior to highly emetogenic chemotherapy including cisplatin ≥ 60 mg/m², cyclophosphamide > 1,500 mg/m², and dacarbazine. Corticosteroids were coadministered prophylactically before chemotherapy in 67% of patients. Of the 667 patients, 51% were women, 60% White, and 59% naïve to previous chemotherapy. The mean age was 52 years.
Efficacy Results
The antiemetic activity of Palonosetron Hydrochloride Injection was evaluated during the acute phase (0 to 24 hours) [Table 4], delayed phase (24 to 120 hours) [Table 5], and overall phase (0 to 120 hours) [Table 6] post-chemotherapy in Phase 3 trials.
Table 4: Prevention of Acute Nausea and Vomiting (0 to 24 hours):
Complete Response Rates
Chemotherapy
Study
Treatment
Group
Na
% with Complete Response
p-valueb
97.5% Confidence Interval Palonosetron Hydrochloride Injection minus Comparatorc
Moderately Emetogenic
1
Palonosetron Hydrochloride Injection
0.25 mg
189
81
0.009
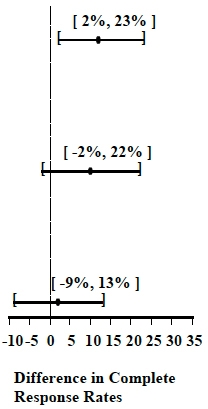
Ondansetron 32 mg I.V.
185
69
2
Palonosetron Hydrochloride Injection
0.25 mg
189
63
NS
Dolasetron 100 mg I.V.
191
53
Highly Emetogenic
3
Palonosetron Hydrochloride Injection
0.25 mg
223
59
NS
Ondansetron 32 mg I.V.
221
57
a Intent-to-treat cohort
b 2-sided Fisher’s exact test. Significance level at α=0.025.
c These studies were designed to show non-inferiority. A lower bound greater than -15%
demonstrates non-inferiority between Palonosetron Hydrochloride Injection and comparator.
These studies show that Palonosetron Hydrochloride Injection was effective in the prevention of acute nausea and vomiting associated with initial and repeat courses of moderately and highly emetogenic cancer chemotherapy. In study 3, efficacy was greater when prophylactic corticosteroids were administered concomitantly. Clinical superiority over other 5-HT3 receptor antagonists has not been adequately demonstrated in the acute phase.
Table 5: Prevention of Delayed Nausea and Vomiting (24 to 120 hours):
Complete Response Rates
Chemotherapy
Study
Treatment Group
Na
% with Complete Response
p-valueb
97.5% Confidence Interval Palonosetron Hydrochloride Injection minus Comparatorc
Moderately Emetogenic
1
Palonosetron Hydrochloride Injection
0.25 mg
189
74
<0.001
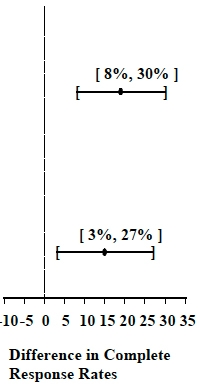
Ondansetron 32 mg I.V.
185
55
2
Palonosetron Hydrochloride Injection
0.25 mg
189
54
0.004
Dolasetron 100 mg I.V.
191
39
a Intent-to-treat cohort
b 2-sided Fisher’s exact test. Significance level at α=0.025.
c These studies were designed to show non-inferiority. A lower bound greater than -15%
demonstrates non-inferiority between Palonosetron Hydrochloride Injection and comparator.
These studies show that Palonosetron Hydrochloride Injection was effective in the prevention of delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy.
Table 6: Prevention of Overall Nausea and Vomiting (0 to 120 hours):
Complete Response Rates
Chemotherapy
Study
Treatment Group
Na
% with Complete Response
p-valueb
97.5% Confidence Interval Palonosetron Hydrochloride Injection minus Comparatorc
Moderately Emetogenic
1
Palonosetron Hydrochloride Injection
0.25 mg
189
69
<0.001
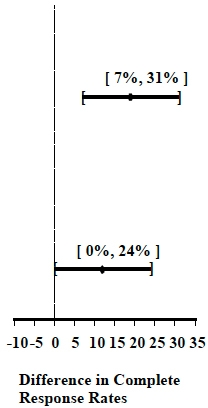
Ondansetron 32 mg I.V.
185
50
2
Palonosetron Hydrochloride Injection
0.25 mg
189
46
0.021
Dolasetron 100 mg I.V.
191
34
a Intent-to-treat cohort
b 2-sided Fisher’s exact test. Significance level at α=0.025.
c These studies were designed to show non-inferiority. A lower bound greater than -15%
demonstrates non-inferiority between Palonosetron Hydrochloride Injection and comparator.
These studies show that Palonosetron Hydrochloride Injection was effective in the prevention of nausea and vomiting throughout the 120 hours (5 days) following initial and repeat courses of moderately emetogenic cancer chemotherapy.
14.2 Chemotherapy-Induced Nausea and Vomiting in Pediatrics
One double-blind, active-controlled clinical trial was conducted in pediatric cancer patients. The total population (N = 327) had a mean age of 8.3 years (range 2 months to 16.9 years) and were 53% male; and 96% white. Patients were randomized and received a 20 mcg/kg (maximum 1.5 mg) intravenous infusion of Palonosetron Hydrochloride Injection 30 minutes prior to the start of emetogenic chemotherapy (followed by placebo infusions 4 and 8 hours after the dose of palonosetron) or 0.15 mg/kg of intravenous ondansetron 30 minutes prior to the start of emetogenic chemotherapy (followed by ondansetron 0.15 mg/kg infusions 4 and 8 hours after the first dose of ondansetron, with a maximum total dose of 32 mg). Emetogenic chemotherapies administered included doxorubicin, cyclophosphamide (< 1,500 mg/m2), ifosfamide, cisplatin, dactinomycin, carboplatin, and daunorubicin. Adjuvant corticosteroids, including dexamethasone, were administered with chemotherapy in 55% of patients.
Complete Response in the acute phase of the first cycle of chemotherapy was defined as no vomiting, no retching, and no rescue medication in the first 24 hours after starting chemotherapy. Efficacy was based on demonstrating non-inferiority of intravenous palonosetron compared to intravenous ondansetron. Non-inferiority criteria were met if the lower bound of the 97.5% confidence interval for the difference in Complete Response rates of intravenous palonosetron minus intravenous ondansetron was larger than -15%. The non-inferiority margin was 15%.
Efficacy Results
As shown in Table 7, intravenous Palonosetron Hydrochloride Injection 20 mcg/kg (maximum 1.5 mg) demonstrated non-inferiority to the active comparator during the 0 to 24 hour time interval.
Table 7: Prevention of Acute Nausea and Vomiting (0 to 24 hours):
Complete Response RatesI.V. Palonosetron
Hydrochloride Injection
20 mcg/kg
(N=165)I.V. Ondansetron
0.15 mg/kg x 3
(N=162)Difference [97.5%
Confidence Interval]*:
I.V. Palonosetron
Hydrochloride Injection
minus I.V. Ondansetron
Comparator59.4% 58.6% 0.36% [-11.7%, 12.4%]
* To adjust for multiplicity of treatment groups, a lower-bound of a
97.5% confidence interval was used to compare to -15%, the negative
value of the non-inferiority margin.
In patients that received Palonosetron Hydrochloride Injection at a lower
dose than the recommended dose of 20 mcg/kg, non-inferiority criteria
were not met. -
16 HOW SUPPLIED/STORAGE AND HANDLING
Palonosetron Hydrochloride Injection 0.25 mg/5 mL (free base) single-dose prefilled syringe is available as follows:
Product
No.
NDC
No.
Strength
Package
673189
63323-673-89
0.25 mg per 5 mL
(0.05 mg per mL)
5 mL single-dose prefilled syringe, individually packaged in cartons of ten.
Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Protect from freezing.
Protect from light.
Discard unused portion.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Instructions for Patients
Patients should be advised to report to their physician all of their medical conditions, including any pain, redness, or swelling in and around the infusion site [see Adverse Reactions (6.3)].
Advise patients of the possibility of serotonin syndrome, especially with concomitant use of Palonosetron Hydrochloride Injection and another serotonergic agent such as medications to treat depression and migraines. Advise patients to seek immediate medical attention if the following symptoms occur: changes in mental status, autonomic instability, neuromuscular symptoms with or without gastrointestinal symptoms [see Warnings and Precautions (5.2)].
Patients should be instructed to read the Patient Information.
Manufactured for:

Lake Zurich, IL 60047Made in Austria
www.fresenius-kabi.com/us
451400A
Issued: June 2018
-
PATIENT PACKAGE INSERT
Patient Information
Palonosetron Hydrochloride (PAL-oh-NOE-se-tron HYE-dro-KLOR-ide) Injection
for Intravenous Use
Read this Patient Information before you receive Palonosetron Hydrochloride Injection and each time you receive Palonosetron Hydrochloride Injection. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
What is Palonosetron Hydrochloride Injection?
Palonosetron Hydrochloride Injection is a prescription medicine called an “antiemetic.”
Palonosetron Hydrochloride Injection is used in adults to help prevent the nausea and vomiting that happens:
right away or later with certain anti-cancer medicines (chemotherapy)
Palonosetron Hydrochloride Injection is used in children 1 month old to less than 17 years of age to help prevent the nausea and vomiting that happens right away with certain anti-cancer medicines (chemotherapy).
It is not known if Palonosetron Hydrochloride Injection is safe and effective in children less than 1 month old to help prevent nausea and vomiting after chemotherapy.
Who should not receive Palonosetron Hydrochloride Injection?
Do not receive Palonosetron Hydrochloride Injection if you are allergic to palonosetron hydrochloride or any of the ingredients in Palonosetron Hydrochloride Injection. See the end of this leaflet for a complete list of ingredients in Palonosetron Hydrochloride injection.
What should I tell my doctor before receiving Palonosetron Hydrochloride Injection?
Before receiving Palonosetron Hydrochloride Injection, tell your doctor about all of your medical conditions, including if you:
have had an allergic reaction to another medicine for nausea or vomiting
are pregnant or plan to become pregnant. It is not known if Palonosetron Hydrochloride Injection will harm your unborn baby.
are breastfeeding or plan to breastfeed. It is not known if Palonosetron Hydrochloride Injection passes into your breast milk. You and your doctor should decide if you will receive Palonosetron Hydrochloride Injection if you breastfeed.
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
How will I receive Palonosetron Hydrochloride Injection?
Palonosetron Hydrochloride Injection will be given to you in your vein by intravenous (I.V.) injection.
Palonosetron Hydrochloride Injection is usually given about 30 minutes before you receive your anti-cancer medicine (chemotherapy).
What are the possible side effects of Palonosetron Hydrochloride Injection?
Palonosetron Hydrochloride Injection can cause allergic reactions that can sometimes be serious. Tell your doctor or nurse right away if you have any of the following symptoms of a serious allergic reaction with Palonosetron Hydrochloride Injection:
hives
swollen face
breathing trouble
chest pain
The most common side effects of Palonosetron Hydrochloride Injection in adults are headache and constipation.
These are not all the possible side effects from Palonosetron Hydrochloride Injection. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Palonosetron Hydrochloride Injection
Medicines are sometimes prescribed for purposes other than those listed in a Patient
Information leaflet. You can ask your doctor or pharmacist for information about Palonosetron Hydrochloride Injection that is written for health professionals.
What are the ingredients in Palonosetron Hydrochloride Injection?
Active ingredient: palonosetron hydrochloride
Inactive ingredients: mannitol, disodium edetate, and citrate buffer in water
Manufactured for:

Lake Zurich, IL 60047
Made in Austria
For more information, go to www.fresenius-kabi.com/us or call 1-800-551-7176
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: June 2018
451401A
-
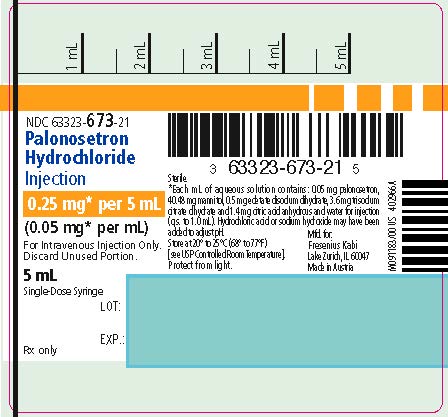
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Palonosetron Hydrochloride 0.25 mg Single-Dose Prefilled Syringe Label
NDC 63323-673-21
Palonosetron Hydrochloride Injection
0.25 mg* per 5 mL
(0.05 mg* per mL)
For Intravenous Injection Only.
Discard Unused Portion.
5 mL
Single-Dose Syringe
Rx only
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Palonosetron Hydrochloride 0.25 mg Single-Dose Prefilled Syringe Individual Carton Panel
NDC 63323-673-21
Palonosetron Hydrochloride Injection
0.25 mg* per 5 mL
(0.05 mg* per mL)
For Intravenous Injection Only.
Discard Unused Portion. Rx only
1x 5 mL Single-Dose Syringe
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Palonosetron Hydrochloride 0.25 mg Single-Dose Prefilled Syringe Carton Panel
NDC 63323-673-89
Palonosetron Hydrochloride Injection
0.25 mg* per 5 mL
(0.05 mg* per mL)
For Intravenous Injection Only.
10 x 5 mL
Single-Dose SyringesRx only

-
INGREDIENTS AND APPEARANCE
PALONOSETRON
palonosetron injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63323-673 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PALONOSETRON HYDROCHLORIDE (UNII: 23310D4I19) (PALONOSETRON - UNII:5D06587D6R) PALONOSETRON 0.25 mg in 5 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 202.4 mg in 5 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 2.5 mg in 5 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 18 mg in 5 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 7 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-673-89 10 in 1 CARTON 09/19/2018 1 NDC: 63323-673-21 5 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206801 09/19/2018 Labeler - Fresenius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations Fresenius Kabi Austria 300206604 ANALYSIS(63323-673) , MANUFACTURE(63323-673) , PACK(63323-673)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.