KARBINAL ER- carbinoxamine maleate suspension, extended release
Karbinal by
Drug Labeling and Warnings
Karbinal by is a Prescription medication manufactured, distributed, or labeled by Aytu Therapeutics, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KARBINAL ® ER safely and effectively. See full prescribing information for KARBINAL ® ER.
Karbinal ® ER (carbinoxamine maleate) extended-release oral suspension
Initial U.S. Approval: 1953INDICATIONS AND USAGE
Karbinal ER (carbinoxamine maleate) Extended-release Oral Suspension is an H 1 receptor antagonist indicated for the symptomatic treatment of:
- Seasonal and perennial allergic rhinitis ( 1)
- Vasomotor rhinitis ( 1)
- Allergic conjunctivitis due to inhalant allergens and foods ( 1)
- Mild, uncomplicated allergic skin manifestations of urticaria and angioedema ( 1)
- Dermatographism ( 1)
- As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled ( 1)
- Amelioration of the severity of allergic reactions to blood or plasma ( 1)
DOSAGE AND ADMINISTRATION
Adults and Adolescents 12 years of age and older ( 2):
7.5 mL to 20 mL (6 to 16 mg) every 12 hours
Children 2-11 years of age (approximately 0.2 to 0.4 mg/kg/day) ( 2):
2 to 3 years – 3.75 mL to 5 mL (3 to 4 mg) every 12 hours
4 to 5 years – 3.75 mL to 10 mL (3 to 8 mg) every 12 hours
6 to 11 years – 7.5 mL to 15 mL (6 to 12 mg) every 12 hours
DOSAGE FORMS AND STRENGTHS
Extended-Release Oral Suspension: 4 mg carbinoxamine maleate per 5 mL ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Activities requiring mental alertness: Avoid engaging in hazardous tasks requiring complete mental alertness such as driving or operating machinery. ( 5.2)
- Anticholinergic actions: Use with caution in patients with increased intraocular pressure, narrow angle glaucoma, hyperthyroidism, cardiovascular disease, hypertension, stenosing peptic ulcer, symptomatic prostatic hypertrophy, bladder neck obstruction, pyloroduodenal obstruction. ( 5.3)
- Contains sodium metabisulfite, a sulfite that may cause anaphylaxis including life-threatening or less severe asthmatic episodes in susceptible individuals. ( 5.4)
ADVERSE REACTIONS
Most common adverse reactions are: sedation, sleepiness, dizziness, disturbed coordination, epigastric distress, and thickening of bronchial secretions. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Cerecor, Inc., at 1-866-416-9637 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Because of the higher risk of serious adverse reactions with use of carbinoxamine maleate in infants, Karbinal ER is contraindicated in nursing women. ( 8.3)
- Contraindicated in children younger than 2 years of age. ( 8.4)
- May cause sedation or excitation in young children. ( 8.4)
- May cause dizziness, sedation, and hypotension in elderly patients. Start elderly patients on lower doses and observe closely for confusion and over-sedation. ( 8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Children less than 2 years of age
4.2 Nursing Mothers
4.3 Hypersensitivity
4.4 Monoamine oxidase inhibitors
5 WARNINGS AND PRECAUTIONS
5.1 Pediatric Mortality
5.2 Activities Requiring Mental Alertness
5.3 Concomitant Medical Conditions
5.4 Allergic Reactions due to Sulfites
5.5 Dosing
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
14. CLINICAL STUDIES
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
17.1. Dosing
17.2. Activities Requiring Mental Alertness
17.3. Alcohol, Sedatives, and Tranquilizers
17.4. MAOIs
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Karbinal ER Extended-release Oral Suspension is an H 1 receptor antagonist indicated for the symptomatic treatment of:
- Seasonal and perennial allergic rhinitis
- Vasomotor rhinitis
- Allergic conjunctivitis due to inhalant allergens and foods
- Mild, uncomplicated allergic skin manifestations of urticaria and angioedema
- Dermatographism
- As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled
- Amelioration of the severity of allergic reactions to blood or plasma
-
2 DOSAGE AND ADMINISTRATION
The dosage of Karbinal ER should be individualized based on the severity of the condition and the response of the patient. Start with lower doses and increase as needed and tolerated.
Administer Karbinal ER by the oral route only. Measure Karbinal ER with an accurate milliliter measuring device. A household teaspoon is not an accurate measuring device and could lead to overdosage. A pharmacist can provide an appropriate measuring device and can provide instructions for measuring the correct dose. [see Warnings and Precautions (5.5)]
Adults and Adolescents 12 years of age and older:
7.5 mL to 20 mL (6 to 16 mg) every 12 hours
Children 2 to 11 years of age (approximately 0.2 to 0.4 mg/kg/day):
2 to 3 years: 3.75 mL to 5 mL (3 to 4 mg) every 12 hours
4 to 5 years: 3.75 mL to 10 mL (3 to 8 mg) every 12 hours
6 to 11 years: 7.5 mL to 15 mL (6 to 12 mg) every 12 hours
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Children less than 2 years of age
Karbinal ER is contraindicated in children younger than 2 years of age because deaths have been reported in this age group [see Warnings and Precautions (5.1)].
4.2 Nursing Mothers
Karbinal ER is contraindicated in nursing mothers because of the risk of mortality in infants given carbinoxamine-containing products [see Use in Specific Populations (8.3)].
4.3 Hypersensitivity
Karbinal ER is contraindicated in patients who are hypersensitive to carbinoxamine maleate or any of the inactive ingredients in Karbinal ER [see Warnings and Precautions (5.4)].
4.4 Monoamine oxidase inhibitors
Karbinal ER is contraindicated in patients who are taking monoamine oxidase inhibitors (MAOI) [see Drug Interactions (7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Pediatric Mortality
Deaths have been reported in children less than 2 years of age who were taking carbinoxamine-containing drug products; therefore, Karbinal ER is contraindicated in children younger than 2 years of age.
5.2 Activities Requiring Mental Alertness
Karbinal ER may produce marked drowsiness and impair the mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. Advise patients to avoid engaging in hazardous tasks requiring mental alertness and motor coordination after ingestion of Karbinal ER. Avoid concurrent use of Karbinal ER with alcohol or other central nervous system depressants because additional impairment of central nervous system performance may occur.
5.3 Concomitant Medical Conditions
Karbinal ER has anticholinergic (atropine-like) properties and, therefore, should be used with caution in patients with: increased intraocular pressure, narrow angle glaucoma, hyperthyroidism, cardiovascular disease, hypertension, stenosing peptic ulcer, symptomatic prostatic hypertrophy, bladder neck obstruction, or pyloroduodenal obstruction.
5.4 Allergic Reactions due to Sulfites
Karbinal ER contains sodium metabisulfite, a sulfite that may cause allergic-type reactions, including anaphylaxis and life-threatening or less severe asthmatic episodes, in susceptible individuals. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic individuals.
5.5 Dosing
Advise patients to measure Karbinal ER with an accurate milliliter measuring device. A household teaspoon is not an accurate measuring device and could lead to overdosage. A pharmacist can recommend an appropriate measuring device and can provide instructions for measuring the correct dose.
-
6 ADVERSE REACTIONS
Use of Karbinal ER may result in decreased mental alertness with impaired mental or physical abilities [see Warnings and Precautions (5.2)].
The most frequent adverse reactions include: sedation, sleepiness, dizziness, disturbed coordination, epigastric distress, and thickening of bronchial secretions. In clinical use, younger children and older adults may be particularly sensitive to adverse reactions [see Pediatric Use (8.4) and Geriatric Use (8.5)].
The following adverse reactions, listed by body system, have been identified in case reports and during the use of carbinoxamine in observational studies. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose and throat.
Cardiovascular: Hypotension, headache, palpitations, tachycardia, extrasystoles.
Central Nervous System: Fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesia, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, hysteria, neuritis, convulsions.
Gastrointestinal: Anorexia, nausea, vomiting, diarrhea, constipation.
Hematologic: Hemolytic anemia, thrombocytopenia, agranulocytosis.
Laboratory: Increase in uric acid levels.
Respiratory: Tightness of chest and wheezing, nasal stuffiness.
Urogenital: Urinary frequency, difficult urination, urinary retention, early menses.
-
7 DRUG INTERACTIONS
Use of Karbinal ER is contraindicated in patients who are taking monoamine oxidase inhibitors (MAOIs), which prolong and intensify the anticholinergic (drying) effects of antihistamines.
Avoid use of Karbinal ER with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.) due to additive effects.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Animal reproductive studies have not been conducted with carbinoxamine maleate. It is also not known whether Karbinal ER can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Karbinal ER should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
Because of the risk of mortality in infants given carbinoxamine-containing drugs, use of Karbinal ER is contraindicated in nursing mothers.
8.4 Pediatric Use
Deaths have been reported in children younger than 2 years of age who were taking carbinoxamine-containing drug products. Therefore, Karbinal ER is contraindicated in children younger than 2 years of age and in nursing mothers. Carbinoxamine may diminish mental alertness or produce sedation in children. Paradoxical reactions with excitation are more likely in younger children.
-
10. OVERDOSAGE
Overdosage with carbinoxamine may cause central nervous system depression or stimulation, hallucinations, convulsions, and death. Atropine-like signs and symptoms – dry mouth; fixed, dilated pupils; flushing; and gastrointestinal symptoms may also occur.
The treatment of overdosage consists of discontinuation of Karbinal ER and institution of symptomatic and supportive therapy. Vital signs (including respiration, pulse, blood pressure, and temperature) and EKG should be monitored. Induction of vomiting is not recommended. Activated charcoal should be given and gastric lavage should be considered after ingestion of a potentially life-threatening amount of drug. In the presence of severe anticholinergic effects, physostigmine may be useful. Vasopressors may be used to treat hypotension.
-
11. DESCRIPTION
Each 5 mL of Karbinal ER Extended-release Oral Suspension contains carbinoxamine complexed with polistirex equivalent to 4 mg carbinoxamine maleate and the following inactive ingredients: citric acid anhydrous, strawberry-banana flavor, glycerin, high fructose corn syrup, methylparaben, modified food starch, polysorbate 80, polyvinyl acetate, povidone, propylparaben, purified water, sodium metabisulfite, sodium polystyrene sulfonate, sucrose, triacetin, and xanthan gum.
Carbinoxamine maleate is freely soluble in water. The chemical name is 2-[(4-chlorophenyl)-2-pyridinylmethoxy]- N, N-dimethylethanamine (Z)-2-butenedioate (1:1), which has the following structure:
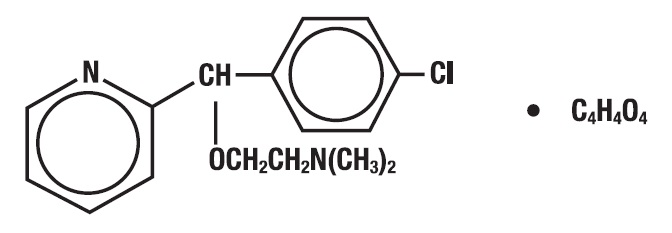
The drug-polistirex complex is formed with the active ingredient (carbinoxamine maleate, USP) and sodium polystyrene sulfonate, USP, which has the following structure:
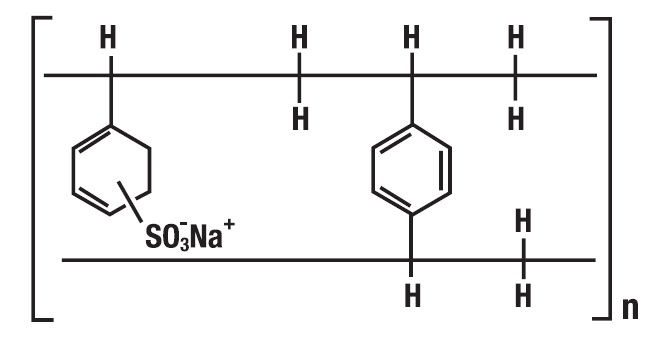
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Carbinoxamine is an H 1 receptor antagonist (antihistamine) that exhibits anticholinergic (drying) and sedative properties.
Antihistamines compete with histamine for receptor sites on effector cells.
12.3 Pharmacokinetics
Karbinal ER after single-dose administration of 16 mg was bioequivalent to the reference carbinoxamine immediate-release oral solution after the administration of two doses of 8 mg six hours apart under fasting conditions. The carbinoxamine mean (SD) peak plasma concentration (C max) was 28.7 (5.3) ng/mL at 6.7 hours after Karbinal ER administration. The plasma half-life of carbinoxamine was 17.0 hours. There was no effect of food on the pharmacokinetic parameters.
Karbinal ER after multiple-dose administration of 16 mg every 12 hours for 8 days was bioequivalent to the reference carbinoxamine immediate-release oral solution after multiple-dose administration of 8 mg every 6 hours. The mean (SD) steady-state C max was 72.9 (24.4) ng/mL at 5.6 hours after Karbinal ER administration. Carbinoxamine mean (SD) minimum plasma concentration at steady-state was 51.8 (20.3) ng/mL.
- 13. NONCLINICAL TOXICOLOGY
-
14. CLINICAL STUDIES
The efficacy and safety of Karbinal ER is based on demonstration of bioequivalence to the immediate-release reference product [see Pharmacokinetics (12.3)].
-
16. HOW SUPPLIED/STORAGE AND HANDLING
Karbinal ER contains 4 mg carbinoxamine maleate per 5 mL. It is a light beige to tan viscous suspension with strawberry-banana flavor and is supplied as follows:
NDC: 23594-101-05 Bottles of 16 fl oz (480 mL)
NDC: 23594-101-01 Bottles of 1 fl oz (30 mL) Physician Samples -
17. PATIENT COUNSELING INFORMATION
17.1. Dosing
Advise patients to measure Karbinal ER with an accurate milliliter measuring device. A household teaspoon is not an accurate measuring device and could lead to overdosage. [see Dosage and Administration (2)].
17.2. Activities Requiring Mental Alertness
Advise patients to use caution when driving a motor vehicle or operating machinery. Karbinal ER may produce marked drowsiness and impair the mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery [see Warnings and Precautions (5.2)].
17.3. Alcohol, Sedatives, and Tranquilizers
Advise patients to avoid the use of alcoholic beverages, sedatives, and tranquilizers while taking Karbinal ER because additional reduction in mental alertness may occur [see Warnings and Precautions (5.2) and Drug Interactions (7)].
17.4. MAOIs
Advise patients to not use MAOIs while taking Karbinal ER. MAOIs may prolong and intensify the anticholinergic (drying) effects [see Contraindications (4.4) and Drug Interactions (7)].
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 480 mL Bottle Label
NDC: 23594-101-05
Karbinal™ ER
(carbinoxamine maleate)
Extended-release
Oral Suspension4 mg/5 ml
Shake Well Before Use
Dose every 12 hours
Dispense with an accurate
milliliter measuring deviceStrawberry Banana Flavored
Rx only 16 fl oz. (480 mL)

-
INGREDIENTS AND APPEARANCE
KARBINAL ER
carbinoxamine maleate suspension, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 23594-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBINOXAMINE MALEATE (UNII: 02O55696WH) (CARBINOXAMINE - UNII:982A7M02H5) CARBINOXAMINE MALEATE 4 mg in 5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM METABISULFITE (UNII: 4VON5FNS3C) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) XANTHAN GUM (UNII: TTV12P4NEE) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) SUCROSE (UNII: C151H8M554) POVIDONE (UNII: FZ989GH94E) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color Score Shape Size Flavor STRAWBERRY (Strawberry Banana) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 23594-101-05 480 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/03/2014 2 NDC: 23594-101-01 2 in 1 CARTON 01/03/2014 2 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022556 01/03/2014 Labeler - Aytu Therapeutics, LLC (117244733)
Trademark Results [Karbinal]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KARBINAL 86568380 4974490 Live/Registered |
TRIS PHARMA, INC 2015-03-18 |
 KARBINAL 85344587 not registered Dead/Abandoned |
TRIS PHARMA, INC 2011-06-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
