MESALAMINE capsule, extended release
mesalamine by
Drug Labeling and Warnings
mesalamine by is a Prescription medication manufactured, distributed, or labeled by Oceanside Pharmaceuticals, Catalent Pharma Solutions, LLC, Carton Service, Incorporated, Bausch Health Companies, Inc., Legacy Pharmaceutical Packaging, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MESALAMINE EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for MESALAMINE EXTENDED-RELEASE CAPSULES.
MESALAMINE EXTENDED-RELEASE CAPSULES
Initial U.S. Approval: 1987RECENT MAJOR CHANGES
- Warnings and Precautions, Phenylketonuria (5.5) 03/2019
INDICATIONS AND USAGE
- Mesalamine extended-release capsules are a locally-acting aminosalicylate indicated for the maintenance of remission of ulcerative colitis in adults. (1)
DOSAGE AND ADMINISTRATION
- Four mesalamine extended-release capsules once daily (1.5 g/day) in the morning with or without food. Do not co-administer with antacids. (2)
DOSAGE FORMS AND STRENGTHS
- Extended-release capsules: 0.375 g (3)
CONTRAINDICATIONS
- Hypersensitivity to salicylates, aminosalicylates, or any component of mesalamine extended-release capsules. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
- The most common adverse reactions (incidence ≥3%) are headache, diarrhea, upper abdominal pain, nausea, nasopharyngitis, flu or flu-like illness, sinusitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Oceanside Pharmaceuticals at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Do not co-administer with antacids (7.1)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
5.2 Mesalamine-Induced Acute Intolerance Syndrome
5.3 Hypersensitivity
5.4 Hepatic Impairment
5.5 Risks in Patients with Phenylketonuria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Adverse Reaction Information from Other Sources
7 DRUG INTERACTIONS
7.1 Antacids
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Ulcerative Colitis
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended dose for maintenance of remission of ulcerative colitis in adult patients is 1.5 g (four mesalamine extended-release capsules) orally once daily in the morning. Mesalamine extended-release capsules may be taken without regard to meals. Mesalamine extended-release capsules should not be co-administered with antacids. An evaluation of renal function is recommended before initiating therapy with mesalamine extended-release capsules.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
Renal impairment, including minimal change nephropathy, acute and chronic interstitial nephritis, and, rarely, renal failure, has been reported in patients given products such as mesalamine extended-release capsules that contain mesalamine or are converted to mesalamine.
It is recommended that patients have an evaluation of renal function prior to initiation of mesalamine extended-release capsules therapy and periodically while on therapy. Exercise caution when using mesalamine extended-release capsules in patients with known renal dysfunction or a history of renal disease.
In animal studies, the kidney was the principal organ for toxicity [see Nonclinical Toxicology (13.2)].
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from a flare of inflammatory bowel disease. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache, and rash. If acute intolerance syndrome is suspected, promptly discontinue treatment with mesalamine extended-release capsules.
5.3 Hypersensitivity
Some patients who have experienced a hypersensitivity reaction to sulfasalazine may have a similar reaction to mesalamine extended-release capsules or to other compounds that contain or are converted to mesalamine.
5.4 Hepatic Impairment
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Caution should be exercised when administering mesalamine extended-release capsules to patients with liver disease.
5.5 Risks in Patients with Phenylketonuria
Phenylalanine can be harmful to patients with phenylketonuria (PKU). Mesalamine extended-release capsules contain phenylalanine, a component of aspartame. Each mesalamine 0.375 g extended-release capsule contains 0.56 mg of phenylalanine. Before prescribing mesalamine extended-release capsules to a patient with PKU, consider the combined daily amount of phenylalanine from all sources, including mesalamine extended-release capsules.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
The data described below reflect exposure to mesalamine extended-release capsules in 557 patients, including 354 exposed for at least 6 months and 250 exposed for greater than one year. Mesalamine extended-release capsules was studied in two placebo-controlled trials (n=367 treated with mesalamine extended-release capsules) and in one open-label, long-term study (n=190 additional patients). The population consisted of patients with ulcerative colitis; the mean age was 47 years, 54% were female, and 93% were white. Patients received doses of mesalamine extended-release capsules 1.5 g administered orally once per day for six months in the placebo-controlled trials and for up to 24 months in the open-label study.
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the two placebo-controlled trials, 59% of mesalamine extended-release capsules-treated patients experienced an adverse reaction compared with 64% of placebo patients. Most adverse reactions with mesalamine extended-release capsules were mild or moderate in severity. Severe adverse reactions occurred in 6% of mesalamine extended-release capsules-treated patients and 5% of placebo-treated patients. Discontinuations due to adverse reactions occurred in 11% of mesalamine extended-release capsules-treated patients and 17% of placebo-treated patients; the most common adverse reaction resulting in study discontinuation was recurrence of ulcerative colitis (mesalamine extended-release capsules 6%, placebo 14%). The most common reactions reported with mesalamine extended-release capsules (≥3%) are shown in Table 1 below.
Table 1: Treatment-Emergent Adverse Reactions During Clinical Trials Occurring in at Least 3% of Mesalamine Extended-Release Capsules-Treated Patients and at a Greater Rate than with Placebo MedDRA Preferred Term Mesalamine Extended-Release Capsules1.5 g/day
N=367Placebo
N=185Headache
11%
8%
Diarrhea
8%
7%
Abdominal Pain Upper
5%
3%
Nausea
4%
3%
Nasopharyngitis
4%
3%
Influenza and Influenza-like Illness
4%
4%
Sinusitis
3%
3%
The following adverse reactions, presented by body system, were reported at a frequency less than 3% in patients treated with mesalamine extended-release capsules for up to 24 months in controlled and open-label trials.
Ear and Labyrinth Disorders: tinnitus, vertigo
Dermatological Disorder: alopecia
Gastrointestinal: abdominal pain lower, rectal hemorrhage
Laboratory Abnormalities: increased triglycerides, decreased hematocrit and hemoglobin
General Disorders and Administration Site Disorders: fatigue
Hepatic: hepatitis cholestatic, transaminases increased
Renal Disorders: creatinine clearance decreased, hematuria
Musculoskeletal: pain, arthralgia
Respiratory: dyspnea
6.2 Adverse Reaction Information from Other Sources
The following adverse reactions have been identified during clinical trials of a product similar to mesalamine extended-release capsules and post approval use of other mesalamine-containing products such as mesalamine extended-release capsules. Because many of these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: lupus-like syndrome, drug fever
Cardiovascular: pericarditis, pericardial effusion, myocarditis
Gastrointestinal: pancreatitis, cholecystitis, gastritis, gastroenteritis, gastrointestinal bleeding, perforated peptic ulcer
Hepatic: jaundice, cholestatic jaundice, hepatitis, liver necrosis, liver failure, Kawasaki-like syndrome including changes in liver enzymes
Hematologic: agranulocytosis, aplastic anemia
Nervous System: intracranial hypertension
Neurological/Psychiatric: peripheral neuropathy, Guillain-Barré syndrome, transverse myelitis
Renal and Urinary: nephrogenic diabetes insipidus
Respiratory/Pulmonary: eosinophilic pneumonia, interstitial pneumonitis
Skin: psoriasis, pyoderma gangrenosum, erythema nodosum
Renal/Urogenital: reversible oligospermia
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B. Reproduction studies with mesalamine have been performed in rats at oral doses up to 320 mg/kg/day (about 1.7 times the recommended human dose based on a body surface area comparison) and rabbits at doses up to 495 mg/kg/day (about 5.4 times the recommended human dose based on a body surface area comparison) and have revealed no evidence of impaired fertility or harm to the fetus due to mesalamine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Mesalamine is known to cross the placental barrier.
8.3 Nursing Mothers
Low concentrations of mesalamine and higher concentrations of its N-acetyl metabolite have been detected in human breast milk. The clinical significance of this has not been determined and there is limited experience of nursing women using mesalamine. Caution should be exercised when mesalamine extended-release capsules are administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of mesalamine extended-release capsules in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of mesalamine extended-release capsules did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing mesalamine extended-release capsules.
Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias, i.e., neutropenia, pancytopenia, in patients who were 65 years or older who were taking mesalamine-containing products such as mesalamine extended-release capsules. Caution should be taken to closely monitor blood cell counts during mesalamine therapy.
Mesalamine is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken when prescribing this drug therapy [see Warnings and Precautions (5.1)].
-
10 OVERDOSAGE
Mesalamine extended-release capsules are an aminosalicylate, and symptoms of salicylate toxicity include hematemesis, tachypnea, hyperpnea, tinnitus, deafness, lethargy, seizures, confusion, or dyspnea. Severe intoxication may lead to electrolyte and blood pH imbalance and potentially to other organ (e.g., renal and liver) involvement. There is no specific antidote for mesalamine overdose; however, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage. This includes prevention of further gastrointestinal tract absorption by emesis and, if necessary, by gastric lavage. Fluid and electrolyte imbalance should be corrected by the administration of appropriate intravenous therapy. Adequate renal function should be maintained. Mesalamine extended-release capsules are a pH-dependent delayed-release product and this factor should be considered when treating a suspected overdose.
-
11 DESCRIPTION
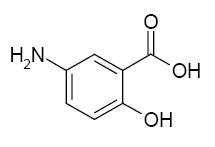
Each mesalamine extended-release capsule is a delayed- and extended-release dosage form for oral administration. Each capsule contains 0.375 g of mesalamine USP (5-aminosalicylic acid, 5-ASA), an anti-inflammatory drug. The structural formula of mesalamine is:
Molecular Weight: 153.14
Molecular Formula: C7H7NO3Each mesalamine extended-release capsule contains granules composed of mesalamine in a polymer matrix with an enteric coating that dissolves at pH 6 and above.
The inactive ingredients of mesalamine extended-release capsules are colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, simethicone emulsion ethyl acrylate/methyl methacrylate copolymer nonoxynol 100 dispersion, hypromellose, methacrylic acid copolymer, talc, titanium dioxide, triethyl citrate, aspartame, anhydrous citric acid, povidone, vanilla flavor, and edible black ink.
Each mesalamine extended-release 0.375 g capsule contains 0.56 mg of phenylalanine. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of mesalamine (5-ASA) is unknown, but appears to be local to the intestinal mucosa rather than systemic. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes and hydroxyeicosatetraenoic acids, is increased in patients with ulcerative colitis, and it is possible that 5-ASA diminishes inflammation by blocking production of arachidonic acid metabolites.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of 5-ASA and its metabolite, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA), were studied after a single and multiple oral doses of 1.5 g mesalamine extended-release capsules in a crossover study in healthy subjects under fasting conditions. In the multiple-dose period, each subject received mesalamine extended-release capsules 1.5 g (4 x 0.375 g capsules) every 24 hours (QD) for 7 consecutive days. Steady state was reached on Day 6 of QD dosing based on trough concentrations.
After single and multiple doses of mesalamine extended-release capsules, peak plasma concentrations were observed at about 4 hours post-dose. At steady state, moderate increases (1.5-fold and 1.7-fold) in systemic exposure (AUC0-24) to 5-ASA and N-Ac-5-ASA were observed when compared with a single-dose of mesalamine extended-release capsules.
Pharmacokinetic parameters after a single dose of 1.5 g mesalamine extended-release capsules and at steady state in healthy subjects under fasting condition are shown in Table 2.
Table 2: Single Dose and Multiple Dose Mean (±SD) Plasma Pharmacokinetic Parameters of Mesalamine (5-ASA) and N-Ac-5-ASA After 1.5 g Mesalamine Extended-Release Capsules Administration in Healthy Subjects
Mesalamine (5-ASA)Single Dose
(n=24)Multiple Dosec
(n=24)a Median (range); b Harmonic mean (pseudo SD); c after 7 days of treatment AUC0-24 (mcg*h/mL)
11±5
17±6
AUC0-inf (mcg*h/mL)
14±5
-
Cmax (mcg/mL)
2.1±1.1
2.7±1.1
Tmax (h)a
4 (2, 16)
4 (2, 8)
t½ (h)b
9±7
10±8
N-Ac-5-ASA
AUC0-24 (mcg*h/mL)
26±6
37±9
AUC0-inf (mcg*h/mL)
51±23
-
Cmax (mcg/mL)
2.8±0.8
3.4±0.9
Tmax (h)a
4 (4, 12)
5 (2, 8)
t½ (h)b
12±11
14±10
In a separate study (n=30), it was observed that under fasting conditions about 32%±11% (mean±SD) of the administered dose was systemically absorbed based on the combined cumulative urinary excretion of 5-ASA and N-Ac-5-ASA over 96 hours post-dose.
The effect of a high fat meal intake on absorption of mesalamine granules (the same granules contained in mesalamine extended-release capsules) was evaluated in 30 healthy subjects. Subjects received 1.6 g of mesalamine granules in sachet (2 x 0.8 g) following an overnight fast or a high fat meal in a crossover study. Under fed conditions, Tmax for both 5-ASA and N-Ac-5-ASA was prolonged by 4 and 2 hours, respectively. A high fat meal did not affect Cmax for 5-ASA, but a 27% increase in the cumulative urinary excretion of 5-ASA was observed with a high fat meal. The overall extent of absorption of N-Ac-5-ASA was not affected by a high fat meal. As mesalamine extended-release capsules and mesalamine granules in sachet were bioequivalent, mesalamine extended-release capsules can be taken without regard to food.
Distribution
In an in vitro study, at 2.5 mcg/mL, mesalamine and N-Ac-5-ASA are 43±6% and 78±1% bound, respectively, to plasma proteins. Protein binding of N-Ac-5-ASA does not appear to be concentration dependent at concentrations ranging from 1 to 10 mcg/mL.
Metabolism
The major metabolite of mesalamine is N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA). It is formed by N-acetyltransferase activity in the liver and intestinal mucosa.
Elimination
Following single and multiple doses of mesalamine extended-release capsules, the mean half-lives were 9 to 10 hours for 5-ASA, and 12 to 14 hours for N-Ac-5-ASA. Of the approximately 32% of the dose absorbed, about 2% of the dose was excreted unchanged in the urine, compared with about 30% of the dose excreted as N-Ac-5-ASA.
In Vitro Drug-Drug Interaction Study
In an in vitro study using human liver microsomes, 5-ASA and its metabolite, N-Ac-5-ASA, were shown not to inhibit the major CYP enzymes evaluated (CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4). Therefore, mesalamine and its metabolite are not expected to inhibit the metabolism of other drugs that are substrates of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP3A4.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Dietary mesalamine was not carcinogenic in rats at doses as high as 480 mg/kg/day, or in mice at 2000 mg/kg/day. These doses are about 2.6 and 5.4 times the recommended human dose of granulated mesalamine capsules of 1.5 g/day (30 mg/kg if 50 kg body weight assumed or 1110 mg/m2), respectively, based on body surface area. Mesalamine was negative in the Ames test, the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test, the sister chromatid exchange assay in the Chinese hamster bone marrow test, and the mouse bone marrow micronucleus test. Mesalamine at oral doses up to 320 mg/kg (about 1.7 times the recommended human dose based on body surface area) was found to have no effect on fertility or reproductive performance in rats.
13.2 Animal Toxicology and/or Pharmacology
Renal Toxicity
Animal studies with mesalamine (13-week and 26-week oral toxicity studies in rats, and 26-week and 52-week oral toxicity studies in dogs) have shown the kidney to be the major target organ of mesalamine toxicity. Oral doses of 40 mg/kg/day (about 0.20 times the human dose, on the basis of body surface area) produced minimal to slight tubular injury, and doses of 160 mg/kg/day (about 0.90 times the human dose, on the basis of body surface area) or higher in rats produced renal lesions including tubular degeneration, tubular mineralization, and papillary necrosis. Oral doses of 60 mg/kg/day (about 1.1 times the human dose, on the basis of body surface area) or higher in dogs also produced renal lesions including tubular atrophy, interstitial cell infiltration, chronic nephritis, and papillary necrosis.
Overdosage
Single oral doses of 800 mg/kg (about 2.2 times the recommended human dose, on the basis of body surface area) and 1800 mg/kg (about 9.7 times the recommended human dose, on the basis of body surface area) of mesalamine were lethal to mice and rats, respectively, and resulted in gastrointestinal and renal toxicity.
-
14 CLINICAL STUDIES
14.1 Ulcerative Colitis
Two similar, randomized, double-blind, placebo-controlled, multi-center studies were conducted in a total of 562 adult patients in remission from ulcerative colitis. The study populations had a mean age of 46 years (11% age 65 years or older), were 53% female, and were primarily white (92%).
Ulcerative colitis disease activity was assessed using a modified Sutherland Disease Activity Index1 (DAI), which is a sum of four subscores based on stool frequency, rectal bleeding, mucosal appearance on endoscopy, and physician’s rating of disease activity. Each subscore can range from 0 to 3, for a total possible DAI score of 12.
At baseline, approximately 80% of patients had a total DAI score of 0 or 1.0. Patients were randomized 2:1 to receive either mesalamine extended-release capsules 1.5 g or placebo once daily in the morning for six months. Patients were assessed at baseline, 1 month, 3 months, and 6 months in the clinic, with endoscopy performed at baseline, at end of study, or if clinical symptoms developed. Relapse was defined as a rectal bleeding subscale score of 1 or more and a mucosal appearance subscale score of 2 or more using the DAI. The analysis of the intent-to-treat population was a comparison of the proportions of patients who remained relapse-free at the end of six months of treatment. For the table below (Table 3) all patients who prematurely withdrew from the study for any reason were counted as relapses.
In both studies, the proportion of patients who remained relapse-free at six months was greater for mesalamine extended-release capsules than for placebo.
Table 3: Percentage of Patients Relapse-Free* Through 6 Months in Mesalamine Extended-Release Capsules Maintenance Studies Mesalamine Extended-Release Capsules
1.5 g/day
% (# no relapse/N)
Placebo
% (# no relapse/N)
Difference
(95% C.I.)
P-value*Relapse counted as rectal bleeding score ≥1 and mucosal appearance score ≥2, or premature withdrawal from study. Study 1
68% (143/209)
51% (49/96)
17% (5.5, 29.2)
<0.001
Study 2
71% (117/164)
59% (55/93)
12% (0, 24.5)
0.046
Examination of gender subgroups did not identify difference in response to mesalamine extended-release capsules among these subgroups. There were too few elderly and too few African-American patients to adequately assess difference in effects in those populations.
The use of mesalamine extended-release capsules for treating ulcerative colitis beyond six months has not been evaluated in controlled clinical trials.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Mesalamine extended-release capsules are available as light blue opaque hard gelatin capsules containing 0.375 g mesalamine and with the letters “G” and “M” on either side of a black band imprinted on the capsule.
NDC: 68682-113-20 Bottles of 120 capsules
Storage:
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F) [see USP Controlled Room Temperature]. -
17 PATIENT COUNSELING INFORMATION
Patients with Phenylketonuria
- Inform patients with phenylketonuria (PKU) or their caregivers that each mesalamine extended-release capsule contains aspartame equivalent to 0.56 mg of phenylalanine, so that the recommended adult dosing provides an equivalent of 2.24 mg of phenylalanine per day.
General Counseling Information
- Instruct patients not to take mesalamine extended-release capsules with antacids, because it could affect the way mesalamine extended-release capsules dissolve.
- Instruct patients to contact a health care provider if they experience a worsening of ulcerative colitis symptoms, because it could be due to a reaction to mesalamine extended-release capsules.
Distributed by:
Oceanside Pharmaceuticals, a division of
Bausch Health US, LLC
Bridgewater, NJ 08807 USAU.S. Patent Number: 8,865,688
© 2019 Bausch Health Companies Inc. or its affiliates
9652001 20002697
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Mesalamine extended-release capsules 120 Capsules, Bottle Label
-
INGREDIENTS AND APPEARANCE
MESALAMINE
mesalamine capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68682-113 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MESALAMINE (UNII: 4Q81I59GXC) (MESALAMINE - UNII:4Q81I59GXC) MESALAMINE 375 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) ASPARTAME (UNII: Z0H242BBR1) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) Product Characteristics Color BLUE (Light Blue) , BLACK (BLACK) Score no score Shape CAPSULE (CAPSULE) Size 23mm Flavor VANILLA (VANILLA) Imprint Code G;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68682-113-20 1 in 1 CARTON 07/19/2018 1 120 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA022301 07/19/2018 Labeler - Oceanside Pharmaceuticals (832011691) Establishment Name Address ID/FEI Business Operations Catalent Pharma Solutions, LLC 829672745 MANUFACTURE(68682-113) Establishment Name Address ID/FEI Business Operations Carton Service, Incorporated 928861723 PACK(68682-113) Establishment Name Address ID/FEI Business Operations Bausch Health Companies, Inc. 253292734 MANUFACTURE(68682-113) , PACK(68682-113)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.