HEPLISAV-B (hepatitis b vaccine- recombinant adjuvanted injection, solution
HEPLISAV-B by
Drug Labeling and Warnings
HEPLISAV-B by is a Other medication manufactured, distributed, or labeled by Dynavax Technologies Corporation, Rhein Biotech GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HEPLISAV-B® safely and effectively. See full prescribing information for HEPLISAV-B.
HEPLISAV-B [Hepatitis B Vaccine (Recombinant), Adjuvanted] Solution for Intramuscular Injection
Initial U.S. Approval: 2017INDICATIONS AND USAGE
HEPLISAV-B is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. HEPLISAV-B is approved for use in adults 18 years of age and older. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
HEPLISAV-B is a solution for injection supplied as a single-dose vial and prefilled syringe. A single dose of HEPLISAV-B is 0.5 mL. (3)
CONTRAINDICATIONS
Severe allergic reaction, such as anaphylaxis, after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV-B, including yeast. (4)
ADVERSE REACTIONS
The most common local reaction was injection site pain (23% - 39%). The most common systemic reactions were fatigue (11% - 17%) and headache (8% - 17%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Dynavax at 1-844-889-8753 or VAERS at 1-800-822-7967 and www.vaers.hhs.gov.
USE IN SPECIFIC POPULATIONS
A pregnancy registry is available for HEPLISAV-B. Women who receive HEPLISAV-B during pregnancy are encouraged to contact 1-844-443-7734. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Regimen
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Managing Allergic Reactions
5.2 Immunocompromised Individuals
5.3 Limitations of Vaccine Effectiveness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Use with Immune Globulin
7.2 Interference with Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Adults on Hemodialysis
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Evaluation of Seroprotection
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage Conditions
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intramuscular administration.
2.2 Administration
HEPLISAV-B is a clear to slightly opalescent, colorless to slightly yellow solution.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
Administer HEPLISAV-B by intramuscular injection in the deltoid region using a sterile needle and syringe.
-
3 DOSAGE FORMS AND STRENGTHS
HEPLISAV-B is a sterile solution for injection available in 0.5 mL single-dose vials and prefilled syringes. [see How Supplied/Storage and Handling (16.1)].
-
4 CONTRAINDICATIONS
Do not administer HEPLISAV-B to individuals with a history of severe allergic reaction (e.g. anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of HEPLISAV-B, including yeast [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Managing Allergic Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of HEPLISAV-B.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
A total of 9597 individuals 18 through 70 years of age received at least 1 dose of HEPLISAV-B in 5 clinical trials conducted in the United States, Canada, and Germany. Data from 3 of these trials are provided below.
Study 1 in Subjects 18 through 55 Years of Age
Study 1 was a randomized, observer-blind, active-controlled, multicenter study in Canada and Germany in which 1810 subjects received at least 1 dose of HEPLISAV-B and 605 subjects received at least 1 dose of Engerix-B® [Hepatitis B Vaccine (Recombinant)]. Enrolled subjects had no history of hepatitis B vaccination or infection. HEPLISAV-B was given as a 2-dose regimen at 0 and 1 month followed by saline placebo at 6 months. Engerix-B was given at 0, 1, and 6 months. In the total study population, the mean age was 40 years; 46% of the subjects were men; 93% were white, 2% black, 3% Asian and 3% Hispanic; 26% were obese, 10% had hypertension, 8% had dyslipidemia, and 2% had diabetes mellitus. These demographic and baseline characteristics were similar in both vaccine groups.
Solicited Local and Systemic Adverse Reactions
Subjects were monitored for local and systemic adverse reactions using diary cards for a 7-day period starting on the day of vaccination. The percentages of subjects who reported local and systemic reactions are shown in Table 1.Table 1
Study 1: Percent of Subjects Who Reported Local or Systemic Reactions
Within 7 Days of VaccinationHEPLISAV-B
%Engerix-B
%Post-Dose* Post-Dose* Reaction 1 2 1 2 3 - * HEPLISAV-B was given as a 2-dose regimen at 0 and 1 month followed by saline placebo at 6 months. Engerix-B was given at 0, 1, and 6 months
- † Redness and swelling ≥ 2.5 cm.
- ‡ Oral temperature ≥ 100.4°F (38.0°C).
Local N=1810 N=1798 N=605 N=603 N=598 Injection Site Pain 38.5 34.8 33.6 24.7 20.2 Injection Site Redness† 4.1 2.9 0.5 1.0 0.7 Injection Site Swelling† 2.3 1.5 0.7 0.5 0.5 Systemic Fatigue 17.4 13.8 16.7 11.9 10.0 Headache 16.9 12.8 19.2 12.3 9.5 Malaise 9.2 7.6 8.9 6.5 6.4 N=1784 N=1764 N=596 N=590 N=561 Fever‡ 1.1 1.5 1.8 1.7 1.8 Note: only subjects having data are included. Clinical trial number: NCT00435812 Unsolicited Adverse Events:
Unsolicited adverse events within 28 days following any injection, including placebo, were reported by 42.0% of HEPLISAV-B recipients and 41.3% of Engerix-B recipients.Serious Adverse Events (SAEs)
Subjects were monitored for serious adverse events for 7 months after the first dose of vaccine. The percentage of subjects reporting serious adverse events was 1.5% in the HEPLISAV-B group and 2.1% in the Engerix-B group. No acute myocardial infarctions were reported. No deaths were reported.Potentially Immune-mediated Adverse Events
Potentially immune-mediated adverse events that occurred within 7 months of the first dose of vaccine were reported in 0.2% (n = 4) of HEPLISAV-B recipients and 0.7% (n = 4) of Engerix-B recipients. The following events were reported in the HEPLISAV-B group in one subject each: granulomatosis with polyangiitis, lichen planus, Guillain-Barré syndrome, and Grave’s disease. The following events were reported in the Engerix-B group in one subject each: Bell’s palsy, Raynaud’s phenomenon, and Grave’s disease. One additional Engerix-B recipient with a history of mixed connective tissue disease had p- ANCA-positive vasculitis.Study 2 in Subjects 40 through 70 Years of Age
Study 2 was a randomized, observer-blind, active-controlled, multicenter study in Canada and the United States in which 1968 subjects received at least 1 dose of HEPLISAV-B and 481 subjects received at least 1 dose of Engerix-B. HEPLISAV-B was given as a 2-dose regimen at 0 and 1 month followed by saline placebo at 6 months. Enrolled subjects had no history of hepatitis B vaccination or infection. Engerix-B was given at 0, 1, and 6 months. In the total population, the mean age was 54 years; 48% of subjects were men; 82% were white, 15% black, 1% Asian and 6% Hispanic; 44% were obese, 30% had hypertension, 30% had dyslipidemia, and 8% had diabetes mellitus. These demographic and baseline characteristics were similar in both vaccine groups.
Solicited Local and Systemic Adverse Reactions
Subjects were monitored for local and systemic adverse reactions using diary cards for a 7-day period starting on the day of vaccination. The percentages of subjects who experienced local and systemic reactions are shown in Table 2.Table 2
Study 2: Percent of Subjects Who Reported Local or Systemic
Reactions Within 7 Days of VaccinationHEPLISAV-B
%Engerix-B
%Post-Dose* Post-Dose* Reaction 1 2 1 2 3 - * HEPLISAV-B was given as a 2-dose regimen at 0 and 1 month followed by saline placebo at 6 months. Engerix-B was given at 0, 1, and 6 months
- † Redness and swelling ≥ 2.5 cm
- ‡ Oral temperature ≥ 100.4°F (38.0°C).
Local N=1952 N=1905 N=477 N=464 N=448 Injection Site Pain 23.7 22.8 18.4 15.9 13.8 Injection Site Redness† 0.9 0.7 0.6 0.2 0.2 Injection Site Swelling† 0.9 0.6 0.6 0.6 0.2 Systemic Fatigue 12.6 10.8 12.8 12.1 9.4 Headache 11.8 8.1 11.9 9.5 8.5 Malaise 7.7 7.0 8.6 7.1 5.1 Myalgia 8.5 6.4 9.6 8.0 4.5 N=1923 N=1887 N=472 N=459 N=438 Fever‡ 0.6 0.6 0.6 0.9 0.7 Note: only subjects having data are included. Clinical Trial Number: NCT01005407 Unsolicited Adverse Events:
Unsolicited adverse events within 28 days following any injection, including placebo, were reported by 35.4% of HEPLISAV-B recipients and 36.2% of Engerix-B recipients.Serious Adverse Events
Subjects were monitored for serious adverse events for 12 months after the first dose of vaccine. The percentage of subjects reporting serious adverse events was 3.9% in the HEPLISAV-B group and 4.8% in the Engerix-B group. Acute myocardial infarction occurred in 0.1% (n=2) of HEPLISAV-B recipients and 0.2% (n=1) of Engerix-B recipients.Autoimmune Adverse Events
Subjects were monitored for the occurrence of new-onset potentially immune-mediated adverse events for 12 months after the first dose of vaccine. Events were adjudicated as to whether they were autoimmune by an external group of experts blinded to treatment assignment. As determined by the adjudicators, new-onset autoimmune adverse events were reported in 0.2% (n=3) of HEPLISAV-B recipients: two subjects with hypothyroidism and one subject with vitiligo. None of these events was considered related to vaccination by the expert group. No new-onset autoimmune adverse events were reported in the Engerix-B group. Although not referred to the external group of experts, one HEPLISAV-B recipient was determined to have Tolosa-Hunt syndrome which is presumed to have an immune-mediated etiology. This event was not considered related to vaccination.Deaths
One subject (0.05%) died of a pulmonary embolism in the HEPLISAV-B group and 1 subject (0.2%) died of heart failure in the Engerix-B group. Neither death was considered related to vaccination.Study 3 in Subjects 18 through 70 Years of Age
Study 3 was a randomized, observer-blind, active-controlled, multicenter study in the United States in which 5587 subjects received at least 1 dose of HEPLISAV-B and 2781 subjects received at least 1 dose of Engerix-B. Enrolled subjects had no history of hepatitis B vaccination or infection. HEPLISAV-B was given as a 2-dose regimen at 0 and 1 month followed by saline placebo at 6 months. Engerix-B was given at 0, 1, and 6 months. In the total study population, the mean age was 50 years; 51% were men; 71% were white, 26% black, 1% Asian, and 9% Hispanic; 48% were obese, 36% had hypertension, 32% had dyslipidemia, and 14% had type 2 diabetes mellitus. These demographic and baseline characteristics were similar in both vaccine groups.
Unsolicited Medically-Attended Adverse Events
Subjects were monitored for unsolicited medically-attended adverse events, those for which a subject sought medical care, for 13 months after the first dose of vaccine. Overall, medically-attended adverse events were reported in 46.0% of HEPLISAV-B recipients and 46.2% of Engerix-B recipients. Herpes zoster was reported in 0.7% of HEPLISAV-B recipients and 0.3% of Engerix-B recipients. Unsolicited medically-attended adverse events within 28 days following any injection, including placebo, were reported by 20.1% of both HEPLISAV-B and Engerix-B recipients.Serious Adverse Events
Subjects were monitored for serious adverse events for 13 months after the first dose of vaccine. The percentage of subjects who reported serious adverse events was 6.2% in the HEPLISAV-B group and 5.3% in the Engerix-B group. Acute myocardial infarction (AMI) was reported in 0.25% (n=14) of HEPLISAV-B recipients and 0.04% (n=1) of Engerix-B recipients. An analysis of serious adverse events likely representing myocardial infarction (MI) was conducted using the standard Medical Dictionary for Regulatory Activities (MedDRA) query (SMQ) for MI. This analysis identified a total of 19 HEPLISAV-B subjects (0.3%) and 3 Engerix-B subjects (0.1%) with events included in the SMQ for MI (these events include the 15 reports of AMI). Additional evidence, including information on temporal relationship and baseline risk factors, does not support a causal relationship between HEPLISAV-B administration and AMI. Among the 19 events identified as MI in HEPLISAV-B recipients, three occurred within 14 days, nine occurred within 53-180 days, and seven occurred more than 180 days following any dose of HEPLISAV-B. Among the three events identified as MI in Engerix- B recipients, one each occurred 13, 115, and 203 days following any dose. All 19 HEPLISAV-B recipients and 3 Engerix-B recipients reported one or more baseline risk factors for cardiovascular disease.Autoimmune Adverse Events
Subjects were monitored for the occurrence of new-onset potentially immune-mediated adverse events for 13 months after the first dose of vaccine. Events were adjudicated as to whether they were autoimmune by an external group of experts who were blinded to treatment assignment. As determined by the adjudicators, new-onset autoimmune adverse events were reported in 0.1% (n=4) of HEPLISAV-B recipients [one each of: alopecia areata, polymyalgia rheumatica, ulcerative colitis, and autoimmune thyroiditis (with concurrent diagnosis of papillary thyroid carcinoma)]. None of these events was considered to be related to vaccination by the external experts. No new-onset autoimmune adverse events were reported in the Engerix-B group.Deaths
During the study death was reported in 25 subjects (0.4%) in the HEPLISAV-B group and 7 subjects (0.3%) in the Engerix-B group. No death was considered related to vaccination. - 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to HEPLISAV-B during pregnancy. Women who receive HEPLISAV-B during pregnancy are encouraged to contact 1-844-443-7734.Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In clinically recognized pregnancies in the US general population, the estimated background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20%.
There are no clinical studies of HEPLISAV-B in pregnant women. Available human data on HEPLISAV- B administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
In a developmental toxicity study, 0.3 mL of a vaccine formulation containing 2.5 mcg HBsAg and 3000 mcg cytosine phosphoguanine (CpG) 1018 adjuvant was administered to female rats prior to mating and during gestation. These animal studies revealed no evidence of harm to the fetus due to this vaccine formulation [see Data].
Animal Data
Developmental toxicity studies were conducted in female rats. Animals were administered 0.3 mL of a vaccine formulation containing 2.5 mcg HBsAg and 3000 mcg CpG 1018 adjuvant twice prior to mating, and on gestation days 6 and 18 (a single human dose of HEPLISAV-B contains 20 mcg HBsAg and 3000 mcg CpG 1018 adjuvant). No adverse effects on pre-natal and post-natal development up to the time of weaning were observed. There were no vaccine-related fetal malformations or variations observed.
8.2 Lactation
Risk Summary
It is not known whether HEPLISAV-B is excreted in human milk. Data are not available to assess the effects of HEPLISAV-B on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for HEPLISAV-B and any potential adverse effects on the breastfed child from HEPLISAV- B or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Safety and effectiveness of HEPLISAV-B have not been established in individuals less than 18 years of age.
8.5 Geriatric Use
Clinical trials included 909 adults 65 through 70 years of age who received HEPLISAV-B.
Among subjects who received HEPLISAV-B, a seroprotective level of antibody to HBsAg was achieved in 90% of those 65 through 70 years of age compared to 96% of those aged 18 through 64 years of age.
Safety and effectiveness of HEPLISAV-B in adults older than 70 years of age were extrapolated from findings in subjects younger than 70 years of age.
-
11 DESCRIPTION
HEPLISAV-B [Hepatitis B Vaccine (Recombinant), Adjuvanted] is a sterile solution for intramuscular injection.
The HBsAg is expressed in a recombinant strain of Hansenula polymorpha yeast. The fermentation process involves growth of the recombinant H. polymorpha on chemically-defined fermentation media containing vitamins and mineral salts.
The HBsAg is expressed intra-cellularly in the yeast cells. It is released from the yeast cells by cell disruption and purified by a series of physicochemical steps. Each dose may contain residual amounts of yeast protein (≤5.0% of total protein), yeast DNA (<20 picogram), and deoxycholate (<0.9 ppm) from the HBsAg manufacturing process.
HEPLISAV-B is prepared by combining the purified HBsAg together with the CpG 1018 adjuvant, a 22-mer phosphorothioate linked oligodeoxynucleotide in a phosphate buffered saline (sodium chloride, 9.0 mg/mL; sodium phosphate, dibasic dodecahydrate, 1.75 mg/mL; sodium phosphate, monobasic dihydrate, 0.48 mg/mL; and polysorbate 80, 0.1 mg/mL).
Each 0.5-mL dose is formulated to contain 20 mcg of HBsAg and 3000 mcg of CpG 1018 adjuvant.
HEPLISAV-B is available in vials and prefilled syringes. The tip caps and stoppers of the prefilled syringes and vial stoppers are not made with natural rubber latex.
HEPLISAV-B is formulated without preservatives. [see How Supplied/Storage and Handling (16)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Infection with hepatitis B virus can have serious consequences including acute massive hepatic necrosis and chronic active hepatitis. Chronically infected persons are at increased risk for cirrhosis and hepatocellular carcinoma.
Antibody concentrations ≥10 mIU/mL against HBsAg are recognized as conferring protection against hepatitis B virus infection.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
HEPLISAV-B has not been evaluated for carcinogenicity, mutagenic potential or male infertility in animals. Vaccination of female rats with a vaccine formulation containing 2.5 mcg HBsAg and 3000 mcg CpG 1018 adjuvant had no effect on fertility [see Use in Specific Populations (8)].
-
14 CLINICAL STUDIES
14.1 Evaluation of Seroprotection
The immunogenicity of HEPLISAV-B was evaluated in comparison with a licensed hepatitis B vaccine (Engerix-B) in 3 randomized, active controlled, observer-blinded, multi-center Phase 3 clinical trials of adults. HEPLISAV-B was given as a 2-dose regimen at 0 and 1 months followed by saline placebo at 6 months. Engerix-B was given at 0, 1, and 6 months.
The trials compared the seroprotection rates (% with antibody concentration ≥ 10 mIU/mL) induced by HEPLISAV-B and Engerix-B. Noninferiority was met if the lower bound of the 95% confidence interval of the difference in seroprotection rates (HEPLISAV-B minus Engerix-B) was greater than -10%.
Study 1: Seroprotection in Adults 18 through 55 Years of Age
In Study 1, the immunogenicity population comprised 1511 participants who received HEPLISAV-B and 521 who received Engerix-B. The mean age was 40 years for both groups. The primary analysis compared the seroprotection rate at Week 12 for HEPLISAV-B with that at Week 28 for Engerix-B. Non-inferiority of the seroprotection rate induced by HEPLISAV-B compared to Engerix-B was demonstrated (Table 3).Table 3
Study 1: Seroprotection Rate of HEPLISAV-B and Engerix-B (ages 18 through 55 years)Timepoint HEPLISAV-B
N = 1511Engerix-B
N = 521Difference in SPRs
(HEPLISAV-B minus Engerix-B)SPR (95% CI) SPR (95% CI) Difference (95% CI) - * Noninferiority was met because the lower bound of the 95% confidence interval of the difference in SPRs was greater than -10%.
Week 12 (HEPLISAV-B)
Week 28 (Engerix-B)95% (93.9, 96.1) 81.3% (77.8, 84.6) 13.7% (10.4, 17.5)* CI = confidence interval; N = number of subjects in the analysis population in the group; SPR = seroprotection rate (% with anti-HBs ≥ 10 mIU/mL). Clinical trial number: NCT00435812 Study 2: Seroprotection in Adults 40 through 70 Years of Age
In Study 2, the immunogenicity population comprised 1121 subjects who received HEPLISAV-B and 353 subjects who received Engerix-B. The mean age was 54 years for both groups. The primary analysis compared the seroprotection rate at Week 12 for HEPLISAV-B with that at Week 32 for Engerix-B. Non- inferiority of the seroprotection rate induced by HEPLISAV-B compared to Engerix-B was demonstrated (Table 4).Table 4
Study 2: Seroprotection Rate of HEPLISAV-B and Engerix-B (ages 40 through 70 years)Timepoint HEPLISAV-B
N = 1121Engerix-B
N = 353Difference in SPRs
(HEPLISAV-B minus Engerix-B)SPR (95% CI) SPR (95% CI) Difference (95% CI) - * Noninferiority was met because the lower bound of the 95% confidence interval of the difference in SPRs was greater than -10%.
Week 12 (HEPLISAV-B)
Week 32 (Engerix-B)90.1% (88.2, 91.8) 70.5% (65.5, 75.2) 19.6% (14.7, 24.8)* CI = confidence interval; N = number of subjects in the analysis population in the group; SPR = seroprotection rate (% with anti-HBs ≥ 10 mIU/mL). The SPR following HEPLISAV-B was statistically significantly higher than following Engerix-B (lower bound of the 95% confidence interval of the difference in SPRs was greater than 0%). Clinical trial number: NCT01005407 Study 3: Seroprotection in Adults 18 through 70 Years of Age Including those with Type 2 Diabetes Mellitus
In Study 3, the immunogenicity population comprised 4537 subjects who received HEPLISAV-B and 2289 subjects who received Engerix-B. The mean age was 51 years and 14% of subjects had type 2 diabetes mellitus (defined as having a clinical diagnosis of type 2 diabetes and taking at least an oral or non-insulin injectable hypoglycemic agent and/or insulin).The primary analysis compared the seroprotection rate at Week 28 for HEPLISAV-B (n= 640) with that at Week 28 for Engerix-B (n= 321) in subjects with type 2 diabetes mellitus. Non-inferiority of the seroprotection rate induced by HEPLISAV-B compared to Engerix-B was demonstrated (Table 5).
Table 5
Study 3: Seroprotection Rate of HEPLISAV-B and Engerix-B (subjects with type 2 diabetes mellitus ages 18 through 70 years)Timepoint HEPLISAV-B
N = 640Engerix-B
N = 321Difference in SPRs
(HEPLISAV-B minus Engerix-B)SPR (95% CI) SPR (95% CI) Difference (95% CI) - * Noninferiority was met because the lower bound of the 95% confidence interval of the difference in SPRs was greater than -10%.
Week 28 90.0% (87.4, 92.2) 65.1% (59.6, 70.3) 24.9% (19.3, 30.7)* CI = confidence interval; N = number of subjects in the analysis population in the group; SPR = seroprotection rate (% with anti-HBs ≥ 10 mIU/mL). The SPR following HEPLISAV-B was statistically significantly higher than following Engerix-B (lower bound of the 95% confidence interval of the difference in SPRs was greater than 0%). Clinical trial number: NCT02117934 A secondary analysis compared the seroprotection rate at Week 24 for HEPLISAV-B with that at Week 28 for Engerix-B in the total study population. Non-inferiority of the seroprotection rate induced by HEPLISAV-B compared to Engerix-B was demonstrated (Table 6).
Table 6
Study 3: Seroprotection Rate of HEPLISAV-B and Engerix-B (total study population ages 18 through 70 years)Timepoint HEPLISAV-B
N = 4376Engerix-B
N = 2289Difference in SPRs
(HEPLISAV-B minus Engerix-B)SPR (95% CI) SPR (95% CI) Difference (95% CI) - * Noninferiority was met because the lower bound of the 95% confidence interval of the difference in SPRs was greater than -10%.
Week 24 (HEPLISAV-B)
Week 28 (Engerix-B)95.4% (94.8, 96.0) 81.3% (79.6, 82.8)) 14.2% (12.5, 15.9)* CI = confidence interval; N = number of subjects in the analysis population in the group; SPR = seroprotection rate (% with anti-HBs ≥ 10 mIU/mL). Clinical trial number: NCT02117934 The SPR following HEPLISAV-B was statistically significantly higher than following Engerix-B (lower bound of the 95% confidence interval of the difference in SPRs was greater than 0%.). Another secondary analysis compared the seroprotection rate at Week 24 for HEPLISAV-B with that at Week 28 for Engerix-B, by age group. For each age stratum non-inferiority of the seroprotection rate induced by HEPLISAV-B compared to Engerix-B was demonstrated (Table 7).
Age
(years)Table 7
Study 3: Seroprotection Rates of HEPLISAV-B and Engerix-B* (ages 18 - 70 years)HEPLISAV-B* Engerix-B* Difference in SPRs
(HEPLISAV-B minus Engerix-B)N SPR (95% CI) N SPR (95% CI) Difference (95% CI) - * Week 24 for HEPLISAV-B and Week 28 for Engerix-B
- † Noninferiority was met because the lower bound of the 95% confidence interval of the difference in SPRs was greater than -10%.
18-29 174 100.0% (97.9, 100.0) 99 93.9% (87.3, 97.7) 6.1% (2.8, 12.6)† 30-39 632 98.9% (97.7, 99.6) 326 92.0% (88.5, 94.7) 6.9% (4.2, 10.4)† 40-49 974 97.2% (96.0, 98.2) 518 84.2% (80.7, 87.2) 13.1% (9.9, 16.6)† 50-59 1439 95.2% (94.0, 96.3) 758 79.7% (76.6, 82.5) 15.5% (12.6, 18.7)† 60-70 1157 91.6% (89.9, 93.1) 588 72.6% (68.8, 76.2) 19.0% (15.2, 23.0)† CI = confidence interval; N = number of subjects in the analysis population in the group; SPR = seroprotection rate (% with anti-HBs ≥ 10 mIU/mL). Clinical trial number: NCT02117934 The SPR following HEPLISAV-B was statistically significantly higher than following Engerix-B (lower bound of the 95% confidence interval of the difference in SPRs was greater than 0%). -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
- Vial, 1 dose (0.5 mL) - (NDC number: 43528-002-01)
- Package of 5 single dose vials - (NDC number: 43528-002-05)
- Prefilled syringe, 1 dose (0.5 mL) - (NDC number: 43528-003-01)
- Package of 5 single dose prefilled syringes - (NDC number: 43528-003-05)
The tip caps and stoppers of the prefilled syringes and vial stoppers are not made with natural rubber latex.
-
17 PATIENT COUNSELING INFORMATION
- Inform vaccine recipient of the potential benefits and risks associated with vaccination, as well as the importance of completing the immunization series.
- Emphasize that HEPLISAV-B contains non-infectious purified HBsAg and cannot cause hepatitis B infection.
- Advise vaccine recipient to report any adverse events to their healthcare provider or to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 and www.vaers.hhs.gov.
- Provide the Vaccine Information Statements, which are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
DYNAVAX
Manufactured by:
Dynavax Technologies Corporation
Berkeley, CA 94710 USA© 2018, Dynavax Technologies Corporation.
All rights reserved. -
PRINCIPAL DISPLAY PANEL
Principal Panel - Carton - Vials
Rx only
Hepatitis B Vaccine
(Recombinant),
AdjuvantedHEPLISAV-B™
NDC: 43528-002-05
5 x 0.5 mL
single-dose vialsFor intramuscular
administration onlyDYNAVAX

-
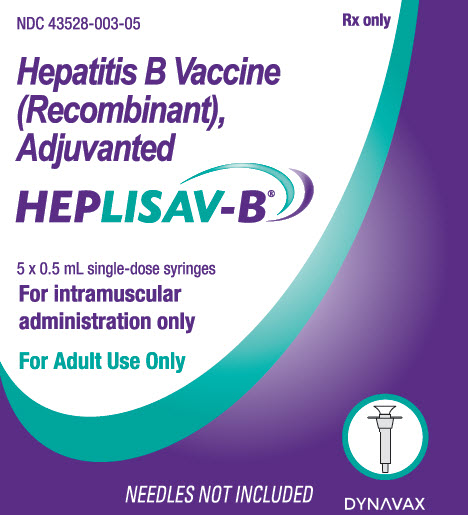
PRINCIPAL DISPLAY PANEL
Principal Panel - Carton - Prefilled Syringes
NDC: 43528-003-05 Rx only
Hepatitis B Vaccine
(Recombinant),
AdjuvantedHEPLISAV-B®
5 x 0.5 mL single-dose syringes
For intramuscular
administration onlyFor Adult Use Only
NEEDLES NOT INCLUDED
DYNAVAX
-
INGREDIENTS AND APPEARANCE
HEPLISAV-B
hepatitis b vaccine (recombinant) adjuvanted injection, solutionProduct Information Product Type VACCINE Item Code (Source) NDC: 43528-002 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN (UNII: XL4HLC6JH6) (HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN - UNII:XL4HLC6JH6) HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN 20 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength 1018 ISS (UNII: 25DT549L0G) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43528-002-05 5 in 1 CARTON 1 NDC: 43528-002-01 0.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125428 01/01/2018 10/17/2019 HEPLISAV-B
hepatitis b vaccine (recombinant) adjuvanted injection, solutionProduct Information Product Type VACCINE Item Code (Source) NDC: 43528-003 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN (UNII: XL4HLC6JH6) (HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN - UNII:XL4HLC6JH6) HEPATITIS B VIRUS SUBTYPE ADW HBSAG SURFACE PROTEIN ANTIGEN 20 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength 1018 ISS (UNII: 25DT549L0G) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43528-003-05 5 in 1 CARTON 1 NDC: 43528-003-01 0.5 mL in 1 SYRINGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125428 01/01/2018 Labeler - Dynavax Technologies Corporation (964173801) Registrant - Dynavax Technologies Corporation (964173801) Establishment Name Address ID/FEI Business Operations Rhein Biotech GmbH 318031242 ANALYSIS(43528-002, 43528-003) , API MANUFACTURE(43528-002, 43528-003)
Trademark Results [HEPLISAV-B]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HEPLISAV-B 98014169 not registered Live/Pending |
Dynavax Technologies Corporation 2023-05-25 |
 HEPLISAV-B 98014166 not registered Live/Pending |
Dynavax Technologies Corporation 2023-05-25 |
 HEPLISAV-B 98014161 not registered Live/Pending |
Dynavax Technologies Corporation 2023-05-25 |
 HEPLISAV-B 87145972 5419134 Live/Registered |
Dynavax Technologies Corporation 2016-08-22 |
 HEPLISAV-B 85840674 not registered Dead/Abandoned |
Dynavax Technologies Corporation 2013-02-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.