CAPLYTA- lumateperone capsule
CAPLYTA by
Drug Labeling and Warnings
CAPLYTA by is a Prescription medication manufactured, distributed, or labeled by Intra-Cellular Therapies, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CAPLYTA safely and effectively. See full prescribing information for CAPLYTA.
CAPLYTA™ (lumateperone) capsules, for oral use
Initial U.S. Approval: 2019WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete boxed warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. CAPLYTA is not approved for the treatment of patients with dementia-related psychosis. (5.1)
INDICATIONS AND USAGE
CAPLYTA is an atypical antipsychotic indicated for the treatment of schizophrenia in adults. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Capsules: 42 mg (3)
CONTRAINDICATIONS
Known hypersensitivity to lumateperone or any components of CAPLYTA. (4)
WARNINGS AND PRECAUTIONS
- Cerebrovascular Adverse Reactions in Elderly Patients with Dementia-Related Psychosis: Increased incidence of cerebrovascular adverse reactions (e.g., stroke and transient ischemic attack). (5.2)
- Neuroleptic Malignant Syndrome: Manage with immediate discontinuation and close monitoring. (5.3)
- Tardive Dyskinesia: Discontinue treatment if clinically appropriate. (5.4)
- Metabolic Changes: Monitor for hyperglycemia/diabetes mellitus, dyslipidemia, and weight gain. (5.5)
- Leukopenia, Neutropenia, and Agranulocytosis: Perform complete blood counts (CBC) in patients with pre-existing low white blood cell count (WBC) or history of leukopenia or neutropenia. Consider discontinuing CAPLYTA if clinically significant decline in WBC occurs in absence of other causative factors. (5.6)
- Orthostatic Hypotension and Syncope: Monitor heart rate and blood pressure and warn patients with known cardiovascular or cerebrovascular disease, and risk of dehydration or syncope. (5.7)
- Seizures: Use cautiously in patients with a history of seizure or with conditions that lower seizure threshold. (5.9)
- Potential for Cognitive and Motor Impairment: Use caution when operating machinery.(5.10)
ADVERSE REACTIONS
Most common adverse reactions in clinical trials (incidence > 5% and greater than twice placebo) were somnolence/sedation and dry mouth. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Intra-Cellular Therapies, Inc. at 1-888-611-4824 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Recommendations for Concomitant Use with CYP3A4 Inducers and Moderate or Strong CYP3A4 Inhibitors
2.3 Dosage Recommendations for Patients with Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
5.2 Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
5.3 Neuroleptic Malignant Syndrome (NMS)
5.4 Tardive Dyskinesia
5.5 Metabolic Changes
5.6 Leukopenia, Neutropenia, and Agranulocytosis
5.7 Orthostatic Hypotension and Syncope
5.8 Falls
5.9 Seizures
5.10 Potential for Cognitive and Motor Impairment
5.11 Body Temperature Dysregulation
5.12 Dysphagia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with CAPLYTA
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/ STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. CAPLYTA is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of CAPLYTA is 42 mg administered orally once daily with food. Dose titration is not required.
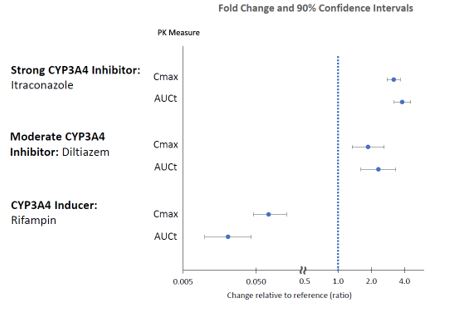
2.2 Dosage Recommendations for Concomitant Use with CYP3A4 Inducers and Moderate or Strong CYP3A4 Inhibitors
Coadministration with CYP3A4 Inducers
Avoid concomitant use of CAPLYTA with CYP3A4 inducers [see Drug Interactions (7.1)].
Coadministration with Moderate or Strong CYP3A4 Inhibitors
Avoid concomitant use of CAPLYTA with moderate or strong CYP3A4 inhibitors [see Drug Interactions (7.1)].
2.3 Dosage Recommendations for Patients with Hepatic Impairment
Avoid use of CAPLYTA in patients with moderate or severe hepatic impairment (Child-Pugh B or C) [see Use in Specific Populations (8.6)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in the drug-treated patients of between 1.6 to 1.7 times that in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in placebo-treated patients. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. CAPLYTA is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning, Warnings and Precautions (5.2)].
5.2 Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
In placebo-controlled trials in elderly subjects with dementia, patients randomized to risperidone, aripiprazole, and olanzapine had a higher incidence of stroke and transient ischemic attack, including fatal stroke. CAPLYTA is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
5.3 Neuroleptic Malignant Syndrome (NMS)
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex, has been reported in association with administration of antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, delirium, and autonomic instability. Additional signs may include elevated creatinine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. If NMS is suspected, immediately discontinue CAPLYTA and provide intensive symptomatic treatment and monitoring.
5.4 Tardive Dyskinesia
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with antipsychotic drugs. The risk appears to be highest among the elderly, especially elderly women, but it is not possible to predict which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of tardive dyskinesia and the likelihood that it will become irreversible increase with the duration of treatment and the cumulative dose. The syndrome can develop after a relatively brief treatment period, even at low doses. It may also occur after discontinuation of treatment.
Tardive dyskinesia may remit, partially or completely, if antipsychotic treatment is discontinued. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome, possibly masking the underlying process. The effect that symptomatic suppression has upon the long-term course of tardive dyskinesia is unknown.
Given these considerations, CAPLYTA should be prescribed in a manner most likely to reduce the risk of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients: 1) who suffer from a chronic illness that is known to respond to antipsychotic drugs; and 2) for whom alternative, effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, use the lowest dose and the shortest duration of treatment producing a satisfactory clinical response. Periodically reassess the need for continued treatment.
If signs and symptoms of tardive dyskinesia appear in a patient on CAPLYTA, drug discontinuation should be considered. However, some patients may require treatment with CAPLYTA despite the presence of the syndrome.
5.5 Metabolic Changes
Antipsychotic drugs have caused metabolic changes, including hyperglycemia, diabetes mellitus, dyslipidemia, and weight gain. Although all of the drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis, hyperosmolar coma or death, has been reported in patients treated with antipsychotics. There have been reports of hyperglycemia in patients treated with CAPLYTA. Assess fasting plasma glucose before or soon after initiation of antipsychotic medication and monitor periodically during long-term treatment.
In pooled data from short-term (4- to 6-week), placebo-controlled trials of adult patients with schizophrenia, mean changes from baseline and the proportion of patients with shifts from normal to greater than normal levels of fasting glucose in patients treated with CAPLYTA were similar to those in patients treated with placebo.
In an uncontrolled open-label trial of CAPLYTA for up to 1 year in patients with stable schizophrenia, the percentages of patients with shifts in fasting glucose and insulin values from normal to high were 8% and 12%, respectively. 4.7% of patients with normal hemoglobin A1c (<6.5%) at baseline developed elevated levels (≥6.5%) post-baseline.
Dyslipidemia
Antipsychotics have caused adverse alterations in lipids. Before or soon after initiation of antipsychotic medications, obtain a fasting lipid profile at baseline and monitor periodically during treatment.
In pooled data from short-term (4- to 6-week), placebo-controlled trials of adult patients with schizophrenia, mean changes from baseline and the proportion of patients with shifts to higher levels of fasting total cholesterol and triglycerides were similar in patients treated with CAPLYTA and placebo.
In an uncontrolled open-label trial of CAPLYTA for up to 1 year in patients with stable schizophrenia, the percentages of patients with a shift from normal to high were 8%, 5%, and 4% for total cholesterol, triglycerides, and LDL cholesterol, respectively.
Weight Gain
Weight gain has been observed with use of antipsychotics. Monitor weight at baseline and frequently thereafter. In pooled data from placebo-controlled trials of adult patients with schizophrenia, mean changes from baseline and the proportion of patients with an increase in weight ≥7% from baseline to end of study was similar in patients treated with CAPLYTA and placebo.
In an uncontrolled open-label trial of CAPLYTA for up to 1 year in patients with stable schizophrenia, the mean change in body weight was approximately -2 kg (SD 5.6) at Day 175 and approximately - 3.2 kg (SD 7.4) at Day 350.
5.6 Leukopenia, Neutropenia, and Agranulocytosis
Leukopenia and neutropenia have been reported during treatment with antipsychotic agents, including CAPLYTA. Agranulocytosis (including fatal cases) has been reported with other agents in the class.
Possible risk factors for leukopenia and neutropenia include pre-existing low white blood cell count (WBC) or absolute neutrophil count (ANC) and history of drug-induced leukopenia or neutropenia. In patients with a pre-existing low WBC or ANC or a history of drug-induced leukopenia or neutropenia, perform a complete blood count (CBC) frequently during the first few months of therapy. In such patients, consider discontinuation of CAPLYTA at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur. Discontinue CAPLYTA in patients with absolute neutrophil count < 1000/mm3 and follow their WBC until recovery.
5.7 Orthostatic Hypotension and Syncope
Atypical antipsychotics cause orthostatic hypotension and syncope. Generally, the risk is greatest during initial dose administration. In these clinical trials the frequencies of orthostatic hypotension for CAPLYTA and placebo were 0.7% and 0%, respectively. The rates of syncope for CAPLYTA and placebo were 0.2% and 0.2%.
Orthostatic vital signs should be monitored in patients who are vulnerable to hypotension (e.g., elderly patients, patients with dehydration, hypovolemia, and concomitant treatment with antihypertensive medications), patients with known cardiovascular disease (history of myocardial infarction, ischemic heart disease, heart failure, or conduction abnormalities), and patients with cerebrovascular disease. CAPLYTA has not been evaluated in patients with a recent history of myocardial infarction or unstable cardiovascular disease. Such patients were excluded from pre-marketing clinical trials.
5.8 Falls
Antipsychotics, including CAPLYTA, may cause somnolence, postural hypotension, and motor and sensory instability, which may lead to falls and, consequently, fractures and other injuries. For patients with diseases, conditions or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and periodically during long-term treatment.
5.9 Seizures
Like other antipsychotic drugs, CAPLYTA may cause seizures. The risk is greatest in patients with a history of seizures or with conditions that lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in older patients.
5.10 Potential for Cognitive and Motor Impairment
CAPLYTA, like other antipsychotics, may cause somnolence and has the potential to impair judgment, thinking, and motor skills. In short-term (i.e., 4- to 6-week) placebo-controlled clinical trials of patients with schizophrenia, somnolence and sedation were reported in 24% of CAPLYTA-treated patients, compared to 10% of placebo-treated patients.
Patients should be cautioned about operating hazardous machinery, including motor vehicles, until they are reasonably certain that therapy with CAPLYTA does not affect them adversely.
5.11 Body Temperature Dysregulation
Atypical antipsychotics may disrupt the body’s ability to reduce core body temperature. Strenuous exercise, exposure to extreme heat, dehydration, and anticholinergic medications may contribute to an elevation in core body temperature; use CAPLYTA with caution in patients who may experience these conditions.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in detail in other sections of the labeling:
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis [see Boxed Warning, Warnings and Precautions (5.1)]
- Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-related Psychosis [see Warnings and Precautions (5.2)]
- Neuroleptic Malignant Syndrome [see Warnings and Precautions (5.3)]
- Tardive Dyskinesia [see Warnings and Precautions (5.4)]
- Metabolic Changes [see Warnings and Precautions (5.5)]
- Leukopenia, Neutropenia, and Agranulocytosis [see Warnings and Precautions (5.6)]
- Orthostatic Hypotension and Syncope [see Warnings and Precautions (5.7)]
- Falls [see Warnings and Precautions (5.8)]
- Seizures [see Warnings and Precautions (5.9)]
- Potential for Cognitive and Motor Impairment [see Warnings and Precautions (5.10)]
- Body Temperature Dysregulation [see Warnings and Precautions (5.11)]
- Dysphagia [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CAPLYTA has been evaluated in 1724 adult patients with schizophrenia exposed to one or more doses. Of these patients, 811 participated in short-term (4- to 6-week), placebo-controlled trials with doses ranging from 14 to 84 mg/day. A total of 329 CAPLYTA-exposed patients had at least 6 months of exposure and 108 had at least 1 year of exposure to the 42-mg dose of CAPLYTA.
There was no single adverse reaction leading to discontinuation that occurred at a rate of >2% in CAPLYTA-treated patients.
The most common adverse reactions (incidence of at least 5% of patients exposed to CAPLYTA and greater than twice the rate of placebo) are somnolence/sedation and dry mouth.
Adverse reactions associated with CAPLYTA (incidence of at least 2% in patients exposed to CAPLYTA and greater than placebo) are shown in Table 1. The following findings are based on the pooled short-term (4- to 6-week), placebo-controlled studies in adult patients with schizophrenia in which CAPLYTA was administered at a daily dose of 42 mg (N=406).
Table 1: Adverse Reactions Reported in >2% of CAPLYTA-Treated Patients in 4- to 6-week Schizophrenia Trials CAPLYTA
42 mg
(N=406)Placebo
(N=412)Somnolence/ Sedation 24% 10% Nausea 9% 5% Dry Mouth 6% 2% Dizziness1 5% 3% Creatine Phosphokinase Increased 4% 1% Fatigue 3% 1% Vomiting 3% 2% Hepatic Transaminases Increased2 2% 1% Decreased Appetite 2% 1% 1 Dizziness, dizziness postural
2 ALT, AST, “hepatic enzymes” increased, or liver function test abnormal
Dystonia
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. Although these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and higher doses of first-generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Extrapyramidal Symptoms
In the 4- to 6-week, placebo-controlled trials, the frequency of reported events related to extrapyramidal symptoms (EPS), including akathisia, extrapyramidal disorder, muscle spasms, restlessness, musculoskeletal stiffness, dyskinesia, dystonia, muscle twitching, tardive dyskinesia, tremor, drooling, and involuntary muscle contractions was 6.7% for CAPLYTA and 6.3% for placebo.
In the 4- to 6-week trials, data were collected using the Simpson Angus Scale (SAS) for EPS (total score ranges from 0 to 40), the Barnes Akathisia Rating Scale (BARS) for akathisia (total score ranges from 0 to 14), and the Abnormal Involuntary Movement Scale (AIMS) for dyskinesia (total score ranges from 0 to 28). The mean changes from baseline for CAPLYTA-treated patients and placebo-treated patients were 0.1 and 0 for the SAS, -0.1 and 0 for the BARS, and 0.1 and 0 for the AIMS, respectively.
-
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with CAPLYTA
Table 2: Clinically Important Drug Interactions with CAPLYTA Moderate or Strong CYP3A4 Inhibitors Clinical Impact Concomitant use of CAPLYTA with moderate or strong CYP3A4 inhibitors increases lumateperone exposure [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions. Intervention Avoid concomitant use of CAPLYTA with moderate or strong CYP3A4 inhibitors [see Dosage and Administration (2.2)]. Examples Moderate inhibitors Amprenavir, ciprofloxacin, cyclosporine, diltiazem, erythromycin, fluconazole, fluvoxamine, verapamil Strong inhibitors Clarithromycin, grapefruit juice, itraconazole, voriconazole, nefazodone, ritonavir, nelfinavir CYP3A4 Inducers Clinical Impact Concomitant use of CAPLYTA with CYP3A4 inducers decreases the exposure of lumateperone [see Clinical Pharmacology (12.3)]. Intervention Avoid concomitant use of CAPLYTA with CYP3A4 inducers [see Dosage and Administration (2.2)]. Examples Carbamazepine, phenytoin, rifampin, St. John’s wort, bosentan, efavirenz, etravirine, modafinil, nafcillin, aprepitant, armodafinil, pioglitazone, prednisone UGT Inhibitors Clinical Impact Concomitant use of CAPLYTA with UGT inhibitors may increase the exposure of lumateperone and/or its metabolites. Intervention Avoid concomitant use of CAPLYTA with UGT inhibitors. Examples Valproic acid, probenecid -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including CAPLYTA, during pregnancy. Healthcare providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or online at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Risk Summary
Neonates exposed to antipsychotic drugs during the third trimester are at risk for extrapyramidal and/or withdrawal symptoms following delivery (see Clinical Considerations). Available data from case reports on CAPLYTA use in pregnant women are insufficient to establish any drug associated risks for birth defects, miscarriage, or adverse maternal or fetal outcomes. There are risks to the mother associated with untreated schizophrenia and with exposure to antipsychotics, including CAPLYTA, during pregnancy (see Clinical Considerations). In animal reproduction studies, no malformations were observed with oral administration of lumateperone to pregnant rats and rabbits during organogenesis at doses up to 2.4 and 9.7 times, respectively, the maximum recommended human dose (MRHD) of 42 mg/day on a mg/m2 basis. When pregnant rats were administered lumateperone during the period of organogenesis through lactation, the number of perinatal deaths of pups was increased at 4.9 times the MRHD, with no adverse effects on pups at 2.4 times the MRHD (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease associated maternal and/or embryo/fetal risk
There is risk to the mother from untreated schizophrenia, including increased risk of relapse, hospitalization, and suicide. Schizophrenia is associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
Fetal/neonatal adverse reactions
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization.
Data
Animal Data
Pregnant rats were treated with oral doses of 3.5, 10.5, 21, and 63 mg/kg/day lumateperone (0.8, 2.4, 4.9, and 14.6 times the MRHD on a mg/m2 basis) during the period of organogenesis. No malformations were observed with lumateperone at doses up to 2.4 times the MRHD. Findings of decreased body weight were observed in fetuses at 4.9 and 14.6 times the MRHD. Findings of incomplete ossification and increased incidences of visceral and skeletal variations were recorded in fetuses at 14.6 times the MRHD, a dose that induced maternal toxicity.
Pregnant rabbits were treated with oral doses of 2.1, 7, and 21 mg/kg/day lumateperone (1.0, 3.2, and 9.7 times the MRHD on a mg/m2 basis) during the period of organogenesis. Lumateperone did not cause adverse developmental effects at doses up to 9.7 times the MRHD.
In a study in which pregnant rats were administered oral doses of 3.5, 10.5, and 21 mg/kg/day lumateperone (0.8, 2.4, and 4.9 times the MRHD on a mg/m2basis) during the period of organogenesis and through lactation, the number of live-born pups was decreased at 2.4 and 4.9 times the MRHD, and early postnatal deaths increased at a dose 4.9 times the MRHD. Impaired nursing and decreased body weight gain in pups were observed at 4.9 times, but not at 2.4 times, the MRHD.
Pregnant rats were treated with a human metabolite of lumateperone (reduced ketone metabolite) at oral doses of 15, 60, and 100 mg/kg/day (1.2, 19, and 27 times the exposure to this metabolite at the MRHD of lumateperone based on AUC plasma exposure) during the period of organogenesis. This metabolite did not cause adverse developmental effects at a dose 1.2 times the exposure at the MRHD of lumateperone; however, it caused an increase in visceral malformations (cleft palate) at 27 times and skeletal malformations at 19 times the exposure at the MRHD of lumateperone, a dose that induced maternal toxicity.
8.2 Lactation
Risk Summary
There are no available data on the presence of lumateperone or its metabolites in human milk or animal milk, the effects on the breastfed infant, or the effects on milk production. Toxicity in animals has been linked to the formation of aniline metabolites of lumateperone [see Nonclinical Toxicology (13.2)]. Although aniline metabolites were not present in (adult) humans at quantifiable levels, it is unknown whether infants exposed to lumateperone will exhibit comparable lumateperone metabolism and elimination pathways as adults. In addition, there are published reports of sedation, failure to thrive, jitteriness, and extrapyramidal symptoms (tremors and abnormal muscle movements) in breastfed infants exposed to antipsychotics. Based on findings of toxicity in animal studies and the potential for serious adverse reactions in the breastfed infant, breastfeeding is not recommended during treatment with lumateperone.
8.3 Females and Males of Reproductive Potential
Infertility
Based on findings from animal studies, lumateperone may impair male and female fertility [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of CAPLYTA have not been established in pediatric patients.
8.5 Geriatric Use
Controlled clinical studies of CAPLYTA did not include any patients aged 65 or older to determine whether or not they respond differently from younger patients.
Antipsychotic drugs increase the risk of death in elderly patients with dementia-related psychosis. CALYPTA is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning, Warnings and Precautions (5.1) and (5.2)].
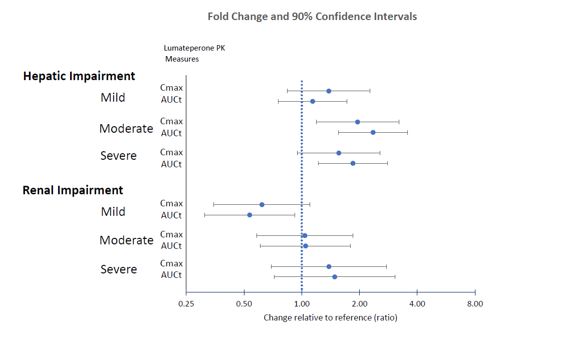
8.6 Hepatic Impairment
Use of CAPLYTA is not recommended for patients with moderate (Child-Pugh class B) to severe hepatic impairment (Child-Pugh class C). Patients with moderate and severe hepatic impairment experienced higher exposure to lumateperone [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
No dosage adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A).
-
10 OVERDOSAGE
No specific antidotes for CAPLYTA are known. In managing overdose, provide supportive care, including close medical supervision and monitoring and consider the possibility of multiple drug involvement. In case of overdose, consult a Certified Poison Control Center (1-800-222-1222 or www.poison.org).
-
11 DESCRIPTION
CAPLYTA capsules contains lumateperone, an atypical antipsychotic, present as lumateperone tosylate salt with the chemical name 4-((6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H,7H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalin-8-yl)-1-(4-fluoro-phenyl)-butan-1-one 4-methylbenzenesulfonate. Its molecular formula is C31H36FN3O4S, and its molecular weight is 565.71 g/mol with the following structure:
CAPLYTA capsules are intended for oral administration. Each CAPLYTA capsule contains 42 mg of lumateperone (equivalent to 60 mg of lumateperone tosylate). Capsules include the following inactive ingredients: croscarmellose sodium, gelatin, magnesium stearate, mannitol, and talc. Colorants include titanium dioxide and FD&C blue #1 and red #3.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of lumateperone in the treatment of schizophrenia is unknown. However, the efficacy of lumateperone could be mediated through a combination of antagonist activity at central serotonin 5-HT2A receptors and postsynaptic antagonist activity at central dopamine D2 receptors.
12.2 Pharmacodynamics
Lumateperone has high binding affinity for serotonin 5-HT2A receptors (Ki = 0.54 nM) and moderate binding affinity for dopamine D2 (Ki = 32 nM) receptors. Lumateperone has moderate binding affinity for serotonin transporters (Ki = 33 nM). Lumateperone also has moderate binding affinity for dopamine D1 (41 nM) and D4 and adrenergic alpha1A and alpha1B receptors (Ki projected at < 100 nM) but has low binding affinity (less than 50% inhibition at 100 nM) for muscarinic and histaminergic receptors.
Cardiac Electrophysiology
QTcF interval was evaluated in a randomized, placebo- and active- (moxifloxacin 400 mg) controlled, four-arm crossover study utilizing concentration-QTc effect modeling in 33 patients with schizophrenia. The placebo-corrected change from baseline QTcF (90% two-sided upper confidence interval) values of 4.9 (8.9) and 15.8 (19.8) ms for the 42 mg and the supratherapeutic dose of 126 mg (three times the recommended daily dosage) CAPLYTA, respectively, administered orally once daily for 5 days.
12.3 Pharmacokinetics
Following once daily oral administration of CAPLYTA, lumateperone steady state is reached in about 5 days. Increase in steady-state exposure is approximately dose-proportional in the range of 21 mg to 56 mg. A large inter-subject variability in lumateperone PK parameters was observed, with coefficients of variation for Cmax (peak plasma concentration) and AUC (area under the concentration vs time curve) ranging from 68% to 97% at steady state.
Absorption
The absolute bioavailability of lumateperone capsules is about 4.4%. Cmax of lumateperone is reached approximately 1-2 hours after CAPLYTA dosing.
Effect of Food
Ingestion of a high-fat meal with CAPLYTA lowers lumateperone mean Cmax by 33% and increases mean AUC by 9%. Median Tmax was delayed about 1 hour (from 1 hour at fasted state to 2 hours in the presence of food).
Distribution
Protein binding of lumateperone is 97.4% at 5 µM (about 70-fold higher than therapeutic concentrations) in human plasma. The volume of distribution of lumateperone following intravenous administration is about 4.1 L/kg.
Elimination
The clearance of lumateperone is approximately 27.9 L/hour and the terminal half-life is about 18 hours after intravenous administration.
Metabolism
Lumateperone is extensively metabolized with more than twenty metabolites identified in vivo. After a single 14C-labeled oral dose, lumateperone and glucuronidated metabolites represent about 2.8% and 51% of the total plasma radioactivity, respectively. In vitro studies show that multiple enzymes, including but not limited to uridine 5'-diphospho-glucuronosyltransferases (UDP-glucuronosyltransferase, UGT) 1A1, 1A4, and 2B15, aldoketoreductase (AKR)1C1, 1B10, and 1C4, and cytochrome P450 (CYP) 3A4, 2C8, and 1A2, are involved in the metabolism of lumateperone.
Excretion
In a human mass‑balance study, 58% and 29% of the radioactive dose was recovered in the urine and feces, respectively. Less than 1% of the dose was excreted as unchanged lumateperone in the urine.
Specific Populations
Effects of hepatic or renal impairment on lumateperone exposure are presented in Figure 1. No clinically significant differences in the pharmacokinetics of lumateperone were observed based on age, sex, or race.
Figure 1: Effects of Intrinsic Factors on Lumateperone Pharmacokinetics
Drug Interaction Studies
Clinical Studies
The effects of other drugs on the exposures of lumateperone are presented in Figure 2.
Figure 2: Effects of Other Drugs on Lumateperone Pharmacokinetics
CYP3A4 substrates: No clinically significant differences in the pharmacokinetics of midazolam (CYP3A4 substrate) or its metabolite 1-hydroxymidazolam were observed when used concomitantly with single or multiple doses of lumateperone in patients with schizophrenia.
In Vitro Studies
Lumateperone showed little to no inhibition of CYP1A2, CYP2C9, CYP2C19, CYP2D6, or CYP3A4/5. It showed no induction of CYP1A2, CYP2B6, or CYP3A4.
Lumateperone did not appear to be a P-gp or BCRP substrate. It showed little to no inhibition of OCT2, OAT1, OAT3, OATP1B3, or OATP1B1.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies were conducted in rats and mice, and results showed no carcinogenic potential in either species.
In Sprague-Dawley rats, males were administered lumateperone (free base) at oral doses of 3.5, 7 or 14 mg/kg/day and females were administered lumateperone at oral doses of 3.5, 10.5, or 21 mg/kg/day for the first 385 days, then doses were reduced for the two higher dose groups so that the females were administered 3.5, 7 or 14 mg/kg/day, respectively, for the duration of the study. In this study the no adverse effect level for neoplastic lesions was determined to be 14 mg/kg/day (84 mg/m2/day) for males and 10.5/7 mg/kg/day (42 mg/m2/day) for females, which are 1.6 times (females) to 3.2 times (males) the MRHD on a mg/m2 basis.
Male and female CD-1 mice were administered lumateperone at oral doses of 3.5, 10.5 or 21 mg/kg/day for the first 35 days, then doses were reduced to 1.4, 4.9, and 14 mg/kg/day, respectively, for the duration of the study. In this study, the no adverse effect level for neoplastic lesions was determined to be 10.5/4.9 mg/kg/day (15 mg/m2/day) for each sex which is 0.6 times the MRHD on a mg/m2 basis.
Mutagenesis
No evidence of mutagenic potential was found in the in vitro bacterial reverse mutation assay (Ames test) and the mouse lymphoma test without metabolic activation. Lumateperone was positive in the Ames test only in the presence of metabolic activation and only in the TA1537 strain and was positive in the mouse lymphoma test only in the presence of metabolic activation and only at high concentrations that inhibited cell growth; together these results were thought to be related to solubility limits and/or nonspecific effects on cellular function. Lumateperone was negative for clastogenic activity in the in vivo micronucleus assay in rats and was not genotoxic in the in vivo Comet assay in rats.
Impairment of Fertility
Female rats were treated with oral doses of 3.5, 10.5, 21 or 42 mg/kg/day lumateperone (free base) (0.8, 2.4, 4.9, and 9.7 times the MRHD on a mg/m2 basis) prior to mating and continuing through conception and implantation. Estrus cycle irregularities were observed at doses ≥10.5 mg/kg/day. Decreases in the median number of corpora lutea and implantation sites, and increases in the number of non-gravid uteruses, were recorded at 42 mg/kg/day. Decreased gestation body weight and body weight gain, and increases in time to mating, were observed at 21 and 42 mg/kg/day.
Male rats were treated with oral doses of 3.5, 10.5, 21 or 42 mg/kg/day lumateperone (0.8, 2.4, 4.9, and 9.7 times the MRHD on a mg/m2 basis) for 9 weeks prior to mating and throughout 14 days of mating. Decreased sperm motility, changes in sperm morphology, reduced epididymal counts, and adverse histopathology changes in testes and epididymides were observed at 21 and 42 mg/kg/day.
13.2 Animal Toxicology and/or Pharmacology
Oral administration of lumateperone caused systemic intracytoplasmic accumulation of pigmented material in dogs, rats, and mice at clinically relevant exposures (AUC). Intracytoplasmic pigmentation appeared to be localized in lysosomes. Accumulation of pigmented material persisted without reversal at the end of 1- to 2-month drug-free periods. Pigmented material was observed in the brain and spinal cord of all three species, and in the heart and eye of rats. Although the composition of the pigmented material was not established, the material is likely polymers or protein adducts formed from aniline metabolites of lumateperone.
In the dog, accumulation of pigmented material in the brain and spinal cord was associated with neuronal degeneration and necrosis, followed by axonal degeneration and histiocytic inflammation after oral administration of lumateperone for up to 9 months. In the rat, accumulation of pigmented material was associated with degenerative changes and signs of an inflammatory response in the spinal cord, peripheral nervous system, eye, and heart after oral administration of lumateperone for up to 2 years. Although overt degenerative changes were not observed in the rat brain, the presence of pigment-containing infiltrating macrophages is consistent with an inflammatory response.
The role of intracytoplasmic pigmented material in causing these lesions was not definitively established; however, the colocalization of pigmented material in tissues with degenerative changes and signs of inflammation is supportive. Alternatively, the aniline metabolites of lumateperone may undergo metabolic activation forming reactive metabolites that contribute to the observed toxicities. The role of intracellular accumulation of lumateperone or its non-aniline metabolites in these toxicities could not be ruled out.
The aniline metabolites thought to be responsible for these toxicities were detected in dogs and rats but were not present in humans at quantifiable levels. Based on all the available evidence, these toxicities do not appear to be relevant to humans.
-
14 CLINICAL STUDIES
CAPLYTA was evaluated for the treatment of schizophrenia in two placebo-controlled trials.
Study 1 (NCT01499563) was a four-week, randomized, double-blind, placebo-controlled, multi-center study in adult patients with a diagnosis of schizophrenia according to the DSM-IV-TR criteria. The primary efficacy measure was the Positive and Negative Syndrome Scale (PANSS) total score. The PANSS is a 30-item scale used to measure symptoms of schizophrenia. Each item is rated by a clinician on a seven-point scale. A score of 1 indicates the absence of symptoms, and a score of 7 indicates extremely severe symptoms. The PANSS total score may range from 30 to 210 with higher scores reflecting greater overall symptom severity.
A total of 335 patients were randomized to receive CAPLYTA 42 mg, CAPLYTA 84 mg (two times the recommended daily dose), an active comparator, or placebo. The study was not designed to allow for efficacy comparison of CAPLYTA and the active comparator. Demographic and baseline disease characteristics were similar for the CAPLYTA, active comparator, and placebo groups. Median age was 42 years (range 20 to 55 years). 17% were female, 19% were Caucasian, and 78% were African American.
Compared to the placebo group, patients randomized to CAPLYTA 42 mg showed a statistically significant reduction from baseline to Day 28 in the PANSS total score. The treatment effect in the CAPLYTA 84 mg group (vs. placebo) was not statistically significant. The results of Study 1 are shown in Table 3.
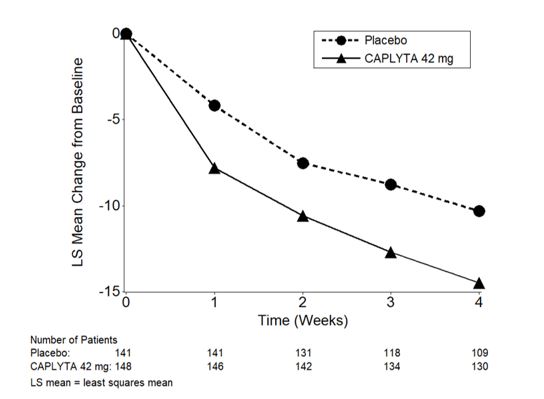
Study 2 (NCT02282761) was a four-week, randomized, double-blind, placebo-controlled, multi-center study in adult patients with a diagnosis of schizophrenia according to the DSM-5 criteria. The primary efficacy measure was the PANSS total score.
A total of 450 patients were randomized to receive CAPLYTA 28 mg (two-thirds the recommended daily dose), CAPLYTA 42 mg, or placebo. Demographic and baseline disease characteristics were similar for the CAPLYTA and placebo groups. Median age was 44 years (range 19 to 60 years); 23% were female, 26% were Caucasian and 66% were African American.
Compared to the placebo group, patients randomized to CAPLYTA 42 mg showed a statistically significant reduction from baseline to Day 28 in the PANSS total score. The treatment effect in the CAPLYTA 28 mg group (vs. placebo) was not statistically significant. The results of Study 2 are shown in Table 3.
Studies 1 and 2 did not include any patients aged 65 or older. Examination of subgroups by sex and race did not suggest differences in response in either study.
Table 3: Primary Efficacy Results for Change from Baseline in PANSS Total Score in Patients with Schizophrenia (Studies 1 and 2) Primary Efficacy Endpoint: PANSS Total Score Study Number
Treatment Group N Mean Baseline Score (SD) LS Mean Change from Baseline (SE) Placebo-subtracted Difference
(95% CI)1 CAPLYTA (42 mg)* 84 88.1 (11.0) -13.2 (1.7) -5.8 (-10.5, -1.1) a Placebo 85 86.3 (13.1) -7.4 (1.7) -- 2 CAPLYTA (42 mg)* 150 90.0 (9.6) -14.5 (1.3) -4.2 (-7.8, -0.6) Placebo 150 89.0 (10.3) -10.3 (1.3) -- The PANSS total score may range from 30 to 210; higher scores reflect greater symptom severity.
SD: standard deviation; SE: standard error; LS Mean: least squares mean; CI: unadjusted confidence interval.
a Difference (drug minus placebo) in LS mean change from baseline not adjusted for sample size increase after unblinded interim analysis.
*Statistically significantly superior to placebo.
Figure 3: Change from Baseline in PANSS Total Score by Time (Weeks) in Patients with Schizophrenia in Study 2.
-
16 HOW SUPPLIED/ STORAGE AND HANDLING
CAPLYTA (lumateperone) capsules are supplied in boxes of 30. Each box contains 3 blister packs of 10 capsules.
Capsule Strength Capsule Color Imprint Codes NDC Code 42 mg Blue cap and opaque white body ITI-007 42 mg 72060-142-30 Store at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Physicians should discuss all relevant safety information with patients, including, but not limited to, the following:
Neuroleptic Malignant Syndrome
Counsel patients about a potentially fatal adverse reaction, Neuroleptic Malignant Syndrome (NMS), that has been reported with administration of antipsychotic drugs. Advise patients, family members, or caregivers to contact the healthcare provider or to report to the emergency room if they experience signs and symptoms of NMS [see Warnings and Precautions (5.3)].
Tardive Dyskinesia
Counsel patients on the signs and symptoms of tardive dyskinesia and to contact their healthcare provider if these abnormal movements occur [see Warnings and Precautions (5.4)].
Metabolic Changes
Educate patients about the risk of metabolic changes, how to recognize symptoms of hyperglycemia and diabetes mellitus, and the need for specific monitoring, including blood glucose, lipids, and weight [see Warnings and Precautions (5.5)].
Leukopenia/Neutropenia
Advise patients with a pre-existing low WBC or a history of drug induced leukopenia/ neutropenia that they should have their CBC monitored while taking CAPLYTA [see Warnings and Precautions (5.6)].
Orthostatic Hypotension and Syncope
Educate patients about the risk of orthostatic hypotension and syncope, especially early in treatment, and also at times of re-initiating treatment [see Warnings and Precautions (5.7)].
Interference with Cognitive and Motor Performance
Caution patients about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that CAPLYTA therapy does not affect them adversely [see Warnings and Precautions (5.10)].
Heat Exposure and Dehydration
Educate patients regarding appropriate care in avoiding overheating and dehydration [see Warnings and Precautions (5.11)].
Concomitant Medications
Advise patients to inform their health care providers of any changes to their current prescription or over-the-counter medications because there is a potential for interactions [see Drug Interactions (7.1)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with CAPLYTA. Advise patients that CAPLYTA used during the third trimester may cause extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder) in the neonate. Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to CAPLYTA during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise females not to breastfeed during treatment with lumateperone [see Use in Specific Populations (8.2)].
Infertility
Advise males and females of reproductive potential that CAPLYTA may impair fertility [see Use in Specific Populations (8.3)].
Distributed by Intra-Cellular Therapies, Inc.
New York, NY 10016
CAPLYTA is a trademark of Intra-Cellular Therapies, Inc.
© 2019 Intra-Cellular Therapies, Inc. All rights reserved
-
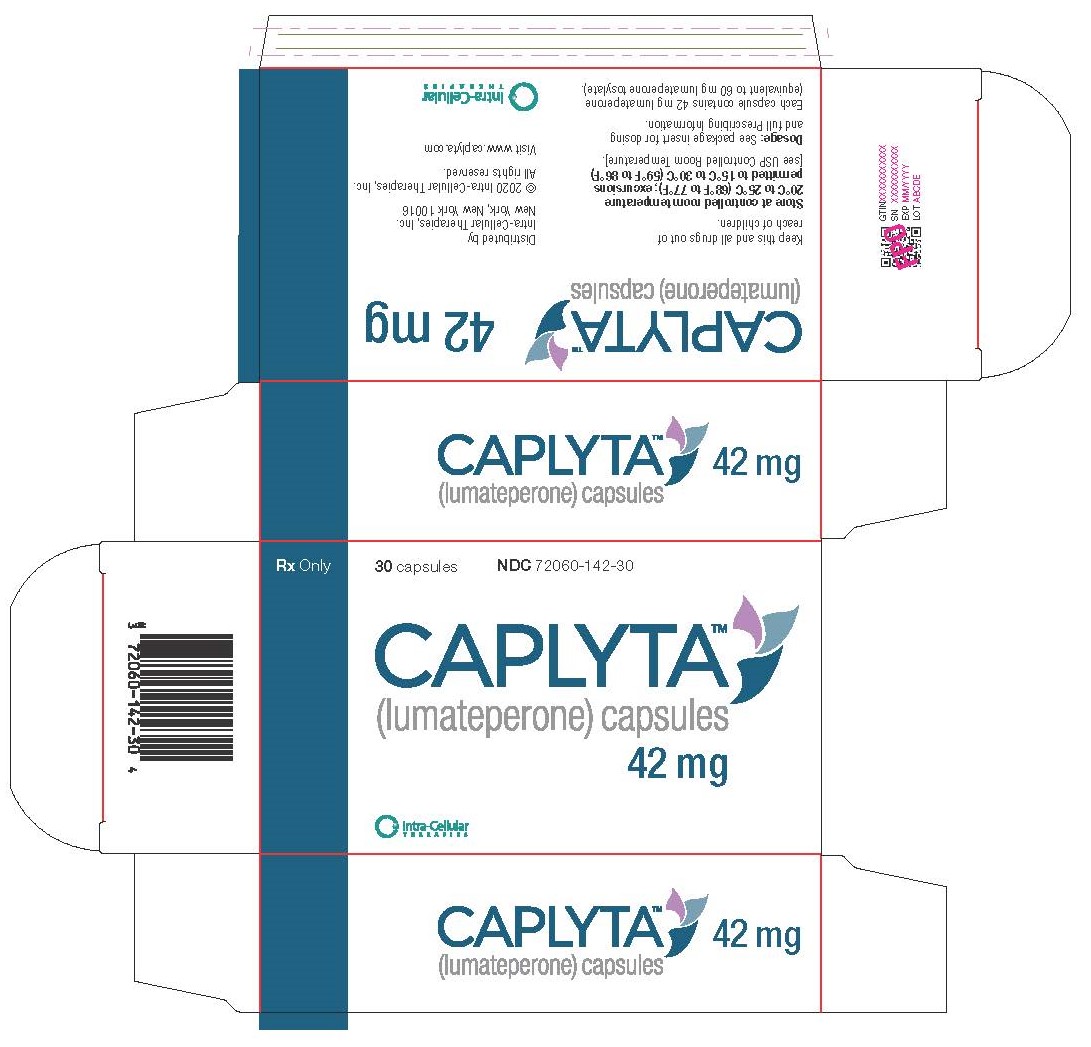
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 42 mg Capsule Blister Pack Carton
Rx Only
30 capsules
NDC: 72060-142-30
CAPLYTA®
lumateperone
42 mg capsulesIntra-Cellular Therapies, Inc.
For additional information, visit www.caplyta.com
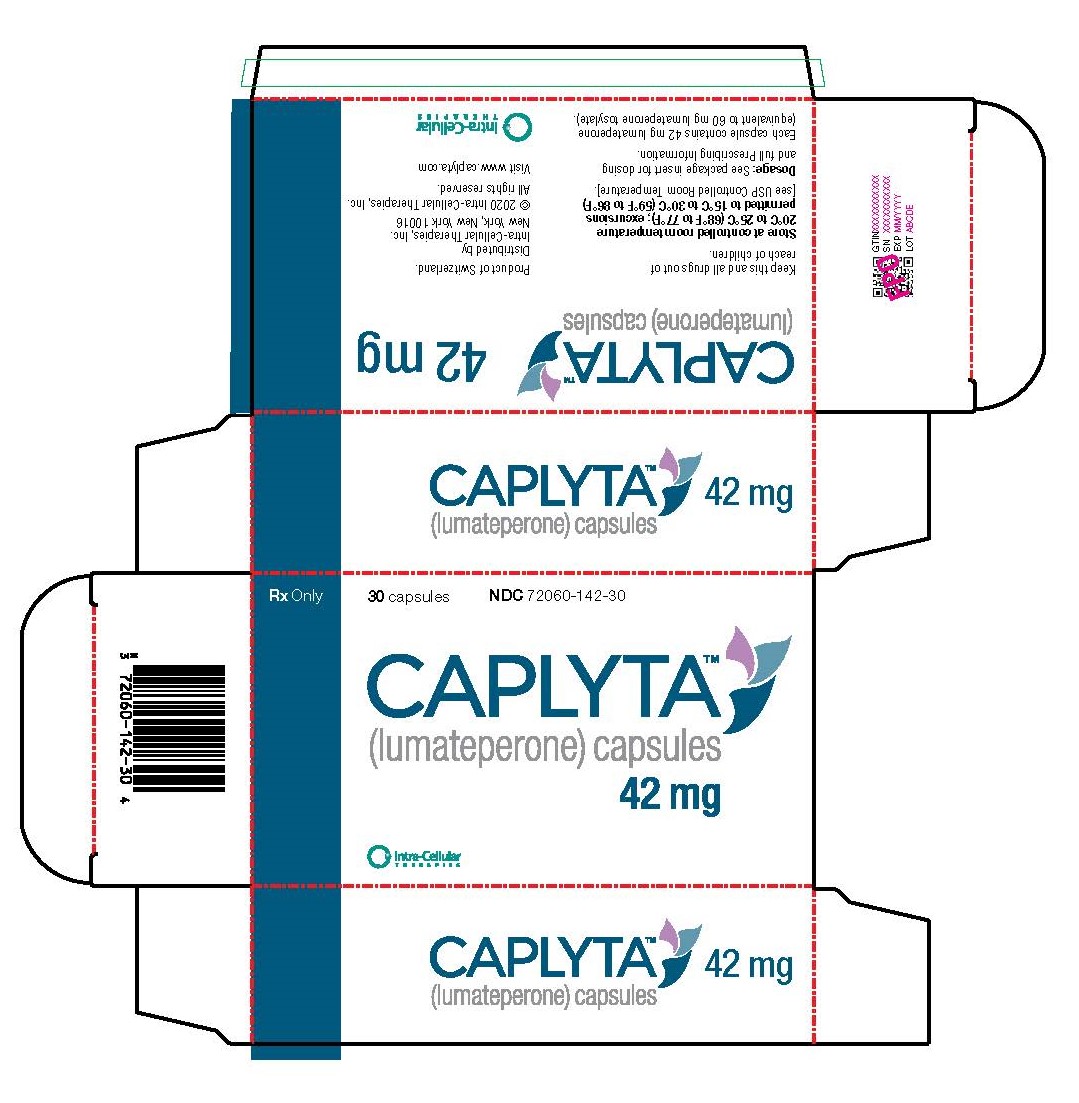
PRINCIPAL DISPLAY PANEL - 42 mg Capsule Blister Pack Carton
Rx Only
30 capsules
NDC: 72060-142-30
CAPLYTA®
lumateperone
42 mg capsulesIntra-Cellular Therapies, Inc.
Product of Switzerland
For additional information, visit www.caplyta.com
-
INGREDIENTS AND APPEARANCE
CAPLYTA
lumateperone capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72060-142 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LUMATEPERONE (UNII: 70BSQ12069) (LUMATEPERONE - UNII:70BSQ12069) LUMATEPERONE 42 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) GELATIN (UNII: 2G86QN327L) Product Characteristics Color BLUE (Blue cap) , WHITE (opaque white body) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code ITI;007;42;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72060-142-30 3 in 1 CARTON 02/01/2020 1 NDC: 72060-142-01 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 72060-142-05 3 in 1 CARTON 02/01/2020 2 NDC: 72060-142-01 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 72060-142-10 1 in 1 CARTON 02/01/2020 3 NDC: 72060-142-01 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209500 02/01/2020 Labeler - Intra-Cellular Therapies, Inc (112765909)
Trademark Results [CAPLYTA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CAPLYTA 88742518 not registered Live/Pending |
INTRA-CELLULAR THERAPIES, INC. 2019-12-30 |
 CAPLYTA 88742498 not registered Live/Pending |
INTRA-CELLULAR THERAPIES, INC. 2019-12-30 |
 CAPLYTA 88340176 not registered Live/Pending |
INTRA-CELLULAR THERAPIES, INC. 2019-03-14 |
 CAPLYTA 87334055 not registered Live/Pending |
INTRA-CELLULAR THERAPIES, INC. 2017-02-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.