sodium iodide- Sodium Iodide, I-123 capsule
Drug Labeling and Warnings
Drug Details [pdf]
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
GE Healthcare (Medi-Physics, Inc.) Sodium Iodide I-123 for diagnostic use is supplied as capsules for oral administration. At calibration time, each capsule has an activity of 3.7 MBq (100 µCi) or 7.4 MBq (200 µCi). Each gelatin capsule contains not more than 20 µg of sodium hydroxide and not more than 1 g of sucrose. Each capsule also contains FD&C Yellow No. 6.
Sodium Iodide I-123 is an odorless compound, freely soluble in water. The I-123 is produced in an accelerator by bombardment of enriched Xe-124 with protons [Xe-124 (p,2n) Cs-123
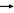 Xe-123
Xe-123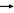 I-123].
I-123].The radionuclidic composition at calibration time is not less than 99.5% I-123 and not more than 0.5% all other nuclides (Te-121, I-125, I-131, I-126, I-124, I-130, I-121 and Na-24). The radionuclidic composition at expiration time is not less than 98.28% I-123 and not more than 1.72% all other nuclides (Te-121, I-125, I-131, I-126, I-124, I-130, I-121 and Na-24).
Molecular formula: Na123I
Molecular Weight: 145.99
- PHYSICAL CHARACTERISTICS
-
EXTERNAL RADIATION
The specific gamma ray constant for I-123 is 11.2 µC/Kg-MBq-hr (1.6 R/hr-mCi) at 1 cm. The first half value thickness of lead (Pb) for I-123 is 0.005 cm. A range of coefficients of attenuation of the radiation emitted by this radionuclide can be achieved by the interposition of various thicknesses of Pb and is shown in Table 2. For example, the use of 1.63 cm of lead will decrease the external radiation exposure by a factor of about 1,000.
Table 2. Radiation Attenuation by Lead Shielding* Shield Thickness (Pb) cm Coefficient of Attenuation - * Method of Calculation: Data supplied by Oak Ridge Associated Universities, Radiopharmaceutical Internal Dose Information Center, 1984.
0.005 0.5 0.10 10-1 0.88 10-2 1.63 10-3 2.48 10-4 To permit correction for the physical decay of I-123, the fractions that remain at selected intervals after the time of calibration are shown in Table 3.
-
CLINICAL PHARMACOLOGY
Sodium Iodide is readily absorbed from the upper gastrointestinal tract. Following absorption, the iodide is distributed primarily within the extracellular fluid of the body. It is concentrated and organically bound by the thyroid and concentrated by the stomach, choroid plexus, and salivary glands. It is also promptly excreted by the kidneys. The normal range of urinary excretion in 24 hours is reported to be 37-75% of the administered dose, varying with thyroid and renal function. The iodide concentrating mechanism of the thyroid, variously termed the iodide "trap" or "pump," accounts for an iodide concentration some 25 times that of the plasma level, but may increase to as much as 500 times under certain conditions.
"Trapped" iodide is oxidized to iodine and organically incorporated so rapidly that the trap contains less than 0.2% free iodide in comparison to organically bound iodine. This process results in a further concentration of iodine in the thyroid gland to about 500 fold that of blood. The iodinated organic compounds consist chiefly of thyroxine (T4) and triiodothyronine (T3), which are bound to thyroglobulin in the follicular colloid. The T4 and T3 are released by enzymatic proteolysis of thyroglobulin into the blood, where they are specifically bound and transported by plasma thyroid binding proteins. These reactions are mostly under the control of anterior-pituitary thyroid stimulating hormone (TSH) and hypothalamic thyroid releasing factor (TRF). Thyroid uptake is usually increased in hyperthyroidism and in goiter with impaired hormone synthesis. Uptake is usually decreased in hypothyroidism and normal or decreased in hyperthyroidism treated with iodide. It should be noted that the uptake of tracer iodine is a function of stable iodide concentration in the serum as well as of alterations in thyroid physiology.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
The contents of the capsule are radioactive. Adequate shielding of the preparation must be maintained at all times. Do not use after the expiration time and date (24 hours after calibration time) stated on the label.
The uptake of I-123 may be decreased by recent administration of iodinated contrast materials, by intake of stable iodine in any form, or by thyroid, antithyroid, and certain other drugs. Accordingly, the patient should be questioned carefully regarding diet, previous medication, and procedures involving radiographic contrast media.
Sodium Iodide I-123, as well as other radioactive drugs, must be handled with care, and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Care should also be taken to minimize radiation exposure to the patient consistent with proper patient management.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility in male or female animals.
Pregnancy Category C
Animal reproduction studies have not been conducted with this drug. It is also not known whether Sodium Iodide I-123 can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Iodide I-123 should be given to a pregnant woman only if clearly needed.
Ideally, examinations using radiopharmaceuticals, especially those elective in nature, in women of childbearing capability should be performed during the first few (approximately 10) days following the onset of menses.
Nursing Mothers
Since I-123 is excreted in human milk, formula-feeding should be substituted for breast-feeding if the agent must be administered to the mother during lactation.
Geriatric Use
Clinical studies of Sodium Iodide I-123 Capsules did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Although rare, reactions associated with the administration of Sodium Iodide isotopes for diagnostic use include, in decreasing order of frequency: nausea, vomiting, chest pain, tachycardia, itching skin, rash and hives.
Allergic type reactions have been reported infrequently following the administration of iodine-containing radiopharmaceuticals.
-
DOSAGE AND ADMINISTRATION
The recommended oral dose range for diagnostic studies of thyroid function in the average adult patient (70 kg) is 3.7-14.8 MBq (100-400 µCi) of Sodium Iodide I-123. The lower portion of the range 3.7 MBq (100 µCi) is recommended for uptake studies alone, and the higher portion 14.8 MBq (400 µCi) for thyroid imaging.
Concentration of I-123 in the thyroid gland should be measured in accordance with standardized procedures. Consideration should be given to the use of proper instrumentation in thyroid imaging with Sodium Iodide I-123. The determination of I-123 concentration in the thyroid gland may be initiated at six hours after administration of the dose.
Use contents of the capsule up to 24 hours after calibration time and date. Thereafter, discard the capsule with its contents. The user should wear waterproof gloves at all times when handling the capsule.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
-
RADIATION DOSIMETRY
The estimated absorbed radiation doses to several organs of an average patient (70 kg) from oral administration of 14.8 MBq (400 µCi) of I-123 supplied by GE Healthcare (Medi-Physics, Inc.), are shown in Table 4 for thyroid uptakes of 5, 15, and 25%.
The figures in Table 4 represent the maximum possible absorbed radiation dose when the recommended dose of GE Healthcare (Medi-Physics, Inc.) Sodium Iodide I-123 is administered at calibration or at expiry.
Table 4. Radiation Dose Estimates for I-123 Sodium Iodide Estimated Radiation Absorbed Dose Maximum Thyroid Uptake (%) TOC* TOE* Organ mGy
14.8 MBqrad
400 µCimGy
14.8 MBqrad
400 µCiI-123 data supplied by Oak Ridge Associated Universities, Radiopharmaceutical Internal Dose Information Center, 1991. - * Concentrations assumed by Oak Ridge for calculations:
Time of Calibration: 99.5% I-123, 0.5% Te-121.
Time of Expiry: 98.3% I-123, 1.7% Te-121.Bladder (voiding interval = 4.8 hrs.) 5 1.4 0.14 1.5 0.15 15 1.3 0.13 1.4 0.14 25 1.2 0.12 1.3 0.13 Stomach Wall 5 0.98 0.098 1.0 0.10 15 0.91 0.091 0.96 0.096 25 0.83 0.083 0.89 0.089 Small Intestine 5 0.26 0.026 0.37 0.037 15 0.25 0.025 0.36 0.036 25 0.23 0.023 0.34 0.034 Liver 5 0.10 0.010 0.15 0.015 15 0.10 0.010 0.15 0.015 25 0.097 0.0097 0.15 0.015 Ovaries 5 0.22 0.022 0.34 0.034 15 0.21 0.021 0.32 0.032 25 0.20 0.020 0.31 0.031 Bone Surfaces 5 0.14 0.014 0.19 0.019 15 0.15 0.015 0.20 0.020 25 0.16 0.016 0.21 0.021 Red Marrow 5 0.10 0.010 0.15 0.015 15 0.10 0.010 0.16 0.016 25 0.10 0.010 0.16 0.016 Testes 5 0.11 0.011 0.19 0.019 15 0.094 0.0094 0.19 0.019 25 0.089 0.0089 0.18 0.018 Thyroid 5 9.5 0.95 9.5 0.95 15 29 2.9 29 2.9 25 51 5.1 51 5.1 Total Body 5 0.11 0.011 0.16 0.016 15 0.12 0.012 0.17 0.017 25 0.13 0.013 0.18 0.018 - * Concentrations assumed by Oak Ridge for calculations:
-
HOW SUPPLIED
Sodium Iodide I-123 capsules for oral administration are supplied as follows:
Product No. 2031 - 3.7 MBq (100 µCi) - orange capsule - NDC: 17156-201-05
Product No. 2032 - 7.4 MBq (200 µCi) - orange/white capsule - NDC: 17156-522-05
At calibration time, each capsule has an activity of 3.7 MBq (100 µCi) or 7.4 MBq (200 µCi). Each gelatin capsule contains not more than 20 µg sodium hydroxide and not more than 1 g of sucrose. Each capsule also contains FD&C Yellow No. 6.
This radiopharmaceutical is licensed by the Illinois Emergency Management Agency for distribution to persons licensed pursuant to 32 Ill. Admin. Code Section 330.260(c) and Section 335, Subpart D, 335.3010 and Subpart E, 335.4010 or under equivalent licenses of an Agreement State or a Licensing State.
The single dose capsule is supplied with a desiccant in a plastic container that is enclosed in a labeled lead shield.
One extra shield label is supplied with each single capsule for attachment to a shielded container other than the one in which the drug product is supplied. The capsule should be stored at room temperature below 30°C, 86°F. The expiration date has been determined to be 24 hours after calibration time and date.
-
DISPOSAL
Users should monitor the amount of radioactivity present prior to disposal of this product. Storage and/or disposal of Sodium Iodide I-123 should be in accordance with the conditions of Agreement State licenses and regulations, or other regulatory agency authorized to license the use of radionuclides.
- SPL UNCLASSIFIED SECTION
-
INGREDIENTS AND APPEARANCE
SODIUM IODIDE
sodium iodide, i-123 capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17156-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Iodide, I-123 (UNII: 29UKX3A616) (Sodium Iodide, I-123 - UNII:29UKX3A616) 100 uCi Inactive Ingredients Ingredient Name Strength Sodium Hydroxide (UNII: 55X04QC32I) 20 ug in 1 Product Characteristics Color ORANGE (orange) Score no score Shape CAPSULE (capsule) Size 16mm Flavor Imprint Code Contains Coating false Symbol false Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17156-201-05 1 in 1 BOX SODIUM IODIDE
sodium iodide, i-123 capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17156-522 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Iodide, I-123 (UNII: 29UKX3A616) (Sodium Iodide, I-123 - UNII:29UKX3A616) 200 uCi Inactive Ingredients Ingredient Name Strength Sodium Hydroxide (UNII: 55X04QC32I) 20 ug in 1 Product Characteristics Color ORANGE (orange) , WHITE (orange) Score no score Shape CAPSULE (capsule) Size 16mm Flavor Imprint Code Contains Coating false Symbol false Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17156-522-05 1 in 1 BOX Labeler - GE Healthcare, Medi-Physics, Inc.
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.