targretin- bexarotene gel
Drug Labeling and Warnings
Drug Details [pdf]
- N/A - Section Title Not Found In Database
-
DESCRIPTION
Targretin® (bexarotene) gel 1% contains bexarotene and is intended for topical application only. Bexarotene is a member of a subclass of retinoids that selectively activate retinoid X receptors (RXRs). These retinoid receptors have biologic activity distinct from that of retinoic acid receptors (RARs).
The chemical name is 4-[1-(5,6,7,8-tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl)ethenyl] benzoic acid, and the structural formula is as follows:
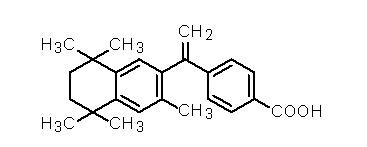
Bexarotene is an off-white to white powder with a molecular weight of 348.48 and a molecular formula of C24H28O2. It is insoluble in water and slightly soluble in vegetable oils and ethanol, USP.
Targretin® gel is a clear gelled solution containing 1.0% (w/w) bexarotene in a base of dehydrated alcohol, USP, polyethylene glycol 400, NF, hydroxypropyl cellulose, NF, and butylated hydroxytoluene, NF.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Bexarotene selectively binds and activates retinoid X receptor subtypes (RXRα, RXRβ, RXRγ). RXRs can form heterodimers with various receptor partners such as retinoic acid receptors (RARs), vitamin D receptor, thyroid receptor, and peroxisome proliferator activator receptors (PPARs). Once activated, these receptors function as transcription factors that regulate the expression of genes that control cellular differentiation and proliferation. Bexarotene inhibits the growth in vitro of some tumor cell lines of hematopoietic and squamous cell origin. It also induces tumor regression in vivo in some animal models. The exact mechanism of action of bexarotene in the treatment of cutaneous T-cell lymphoma (CTCL) is unknown.
Pharmacokinetics
General
Plasma concentrations of bexarotene were determined during clinical studies in patients with CTCL or following repeated single or multiple-daily dose applications of Targretin® gel 1% for up to 132 weeks. Plasma bexarotene concentrations were generally less than 5 ng/mL and did not exceed 55 ng/mL. However, only two patients with very intense dosing regimens (> 40% BSA lesions and QID dosing) were sampled. Plasma bexarotene concentrations and the frequency of detecting quantifiable plasma bexarotene concentrations increased with increasing percent body surface area treated and increasing quantity of Targretin® gel applied. The sporadically-observed and generally low plasma bexarotene concentrations indicated that, in patients receiving doses of low to moderate intensity, there is a low potential for significant plasma concentrations following repeated application of Targretin® gel. Bexarotene is highly bound (>99%) to plasma proteins. The plasma proteins to which bexarotene binds have not been elucidated, and the ability of bexarotene to displace drugs bound to plasma proteins and the ability of drugs to displace bexarotene binding have not been studied (see PRECAUTIONS: Protein Binding). The uptake of bexarotene by organs or tissues has not been evaluated.
Metabolism
Four bexarotene metabolites have been identified in plasma following oral administration of bexarotene: 6- and 7-hydroxy-bexarotene and 6- and 7-oxo-bexarotene. In vitro studies suggest that cytochrome P450 3A4 is the major cytochrome P450 responsible for formation of the oxidative metabolites and that the oxidative metabolites may be glucuronidated. The oxidative metabolites are active in in vitro assays of retinoid receptor activation, but the relative contribution of the parent and any metabolites to the efficacy and safety of Targretin® gel is unknown.
Elimination
The renal elimination of bexarotene and its metabolites was examined in patients with Type 2 diabetes mellitus following oral administration of bexarotene. Neither bexarotene nor its metabolites were excreted in urine in appreciable amounts.
Special Populations
Elderly, Gender, Race: Because of a large number of immeasurable plasma concentrations (< 1ng/mL), any potential pharmacokinetic differences between Special Populations could not be assessed.
Pediatric: Studies to evaluate bexarotene pharmacokinetics in the pediatric population have not been conducted (see PRECAUTIONS: Pediatric Use).
Renal Insufficiency: No formal studies have been conducted with Targretin® gel in patients with renal insufficiency. Urinary elimination of bexarotene and its known metabolites is a minor excretory pathway (<1% of an orally administered dose), but because renal insufficiency can result in significant protein binding changes, pharmacokinetics may be altered in patients with renal insufficiency (seePRECAUTIONS: Renal Insufficiency).
Hepatic Insufficiency: No specific studies have been conducted with Targretin® gel in patients with hepatic insufficiency. Because less than 1% of the dose of oral bexarotene is excreted in the urine unchanged and there is in vitro evidence of extensive hepatic contribution to bexarotene elimination, hepatic impairment would be expected to lead to greatly decreased clearance (see PRECAUTIONS: Hepatic Insufficiency).
Drug-Drug Interactions
No formal studies to evaluate drug interactions with bexarotene or Targretin® gel have been conducted. Bexarotene oxidative metabolites appear to be formed through cytochrome P450 3A4. Drugs that affect levels or activity of cytochrome P450 3A4 may potentially affect the disposition of bexarotene. Concomitant gemfibrozil was associated with increased bexarotene concentrations following oral administration of bexarotene.
Clinical Studies
Targretin® gel was evaluated for the treatment of patients with early stage (Stage IA-IIA) CTCL in one multicenter, open-label, clinical trial as well as in a Phase I-II program (dose-seeking trials with different response criteria than the multicenter trial). These clinical studies enrolled a total of 117 patients.
In the multicenter, open-label clinical trial, Targretin® gel was evaluated for the treatment of patients with early stage CTCL who were refractory to, intolerant to, or reached a response plateau for at least six months on at least two prior therapies. The study was conducted in the U.S., Canada, Europe, and Australia and enrolled a total of 50 patients; 46% of these patients were male, 80% were Caucasian, and the median age was 64 years (range 13 to 85).
Targretin® gel was also evaluated for the treatment of patients with CTCL in a U.S. Phase I-II program involving patients with early stage CTCL. This program enrolled a total of 67 patients; 55% of these patients were male, 85% were Caucasian, and the median age was 61 years (range 30 to 87).
In the multicenter, open-label clinical trial, considering prior systemic, irradiation, and topical treatments, patients had been exposed to a median of 3 prior therapies (range 2-7). All patients failed at least two treatments; the majority (68%) of patients were either refractory to two or more therapies, or were refractory to one therapy and intolerant to at least one therapy.
Patients were treated with Targretin® gel 1% for a planned 16-week period with an option to continue provided that no unacceptable toxicity was occurring.
Tumor response was assessed in the multicenter study by observation of up to five baseline-defined index lesions using a Composite Assessment of Index Lesion Disease Severity (CA). This endpoint was based on a summation of the grades, for all index lesions, of erythema, scaling, plaque elevation, hypopigmentation or hyperpigmentation, and area of involvement. New cutaneous lesions or tumors and extracutaneous disease manifestations were not considered in response or disease progression assessments.
All tumor responses required confirmation over at least two assessments separated by at least four weeks. A partial response was defined as an improvement of at least 50% in the index lesions. A complete clinical response required complete disappearance of the index lesions, but did not require confirmation by biopsy.
Targretin® gel produced an overall response rate of 26% (13/50) with a corresponding exact 95% confidence interval from 14.6% to 40.3% by the Composite Assessment of Index Lesion Severity. For the Stage IA and IB patients, the response rate was 28% (13/47) with a corresponding exact 95% confidence interval from 15.6% to 42.6%. For the Stage II patients the response rate was 0% (0/3). Two percent of patients (1/50) had a clinical complete response. The median time to best response on the Composite Assessment of Index Lesion Severity (n=13) was 85 days (range: 36-154).
The rate of relapse in responding patients by the Composite Assessment of Index Lesion Severity was 23% (3/13) over a median observation period of 149 days (range 56-342). Fourteen patients developed new lesions in untreated areas (14/50; 28%). Four patients developed clinically abnormal lymph nodes (≥ 1cm diam) (4/50; 8%). One patient developed a cutaneous tumor (1/50; 2%).
The Phase I-II program (dose-seeking trials with different response criteria than the multicenter trial) was supportive of the multicenter study results.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Targretin® gel 1% is contraindicated in patients with a known hypersensitivity to bexarotene or other components of the product.
Pregnancy: Category X
Targretin® gel 1% may cause fetal harm when administered to a pregnant woman.
Targretin® gel must not be given to a pregnant woman or a woman who intends to become pregnant. If a woman becomes pregnant while taking Targretin® gel, Targretin® gel must be stopped immediately and the woman given appropriate counseling.
Bexarotene caused malformations when administered orally to pregnant rats during days 7-17 of gestation. Developmental abnormalities included incomplete ossification at 4 mg/kg/day and cleft palate, depressed eye bulge/microphthalmia, and small ears at 16 mg/kg/day. At doses greater than 10 mg/kg/day, bexarotene caused developmental mortality. The no-effect oral dose in rats was 1 mg/kg/day. Plasma bexarotene concentrations in patients with CTCL applying Targretin® gel 1% were generally less than one hundredth the Cmax associated with dysmorphogenesis in rats, although some patients had Cmax levels that were approximately one eighth the concentration associated with dysmorphogenesis in rats.
Women of child-bearing potential should be advised to avoid becoming pregnant when Targretin® gel is used. The possibility that a woman of child-bearing potential is pregnant at the time therapy is instituted should be considered. A negative pregnancy test (e.g., serum beta-human chorionic gonadotropin, beta-HCG) with a sensitivity of at least 50 mIU/L should be obtained within one week prior to Targretin® gel therapy, and the pregnancy test must be repeated at monthly intervals while the patient remains on Targretin® gel. Effective contraception must be used for one month prior to the initiation of therapy, during therapy and for at least one month following discontinuation of therapy; it is recommended that two reliable forms of contraception be used simultaneously unless abstinence is the chosen method. Male patients with sexual partners who are pregnant, possibly pregnant, or who could become pregnant must use condoms during sexual intercourse while applying Targretin® gel and for at least one month after the last dose of drug. Targretin® gel therapy should be initiated on the second or third day of a normal menstrual period. No more than a one month supply of Targretin® gel should be given to the patient so that the results of pregnancy testing can be assessed and counseling regarding avoidance of pregnancy and birth defects can be reinforced.
-
PRECAUTIONS
General: Targretin® gel should be used with caution in patients with a known hypersensitivity to other retinoids. No clinical instances of cross-reactivity have been noted.
Vitamin A Supplementation: In clinical studies, patients were advised to limit vitamin A intake to≤15,000 IU/day. Because of the relationship of bexarotene to vitamin A, patients should be advised to limit vitamin A supplements to avoid potential additive toxic effects.
Photosensitivity: Retinoids as a class have been associated with photosensitivity. In vitro assays indicate that bexarotene is a potential photosensitizing agent. There were no reports of photosensitivity in patients in the clinical studies. Patients should be advised to minimize exposure to sunlight and artificial ultraviolet light during the use of Targretin® gel.
Drug-Drug Interactions
Patients who are applying Targretin® gel should not concurrently use products that contain DEET (N,N-diethyl-m-toluamide), a common component of insect repellent products. An animal toxicology study showed increased DEET toxicity when DEET was included as part of the formulation.
No formal studies to evaluate drug interactions with bexarotene have been conducted. Bexarotene oxidative metabolites appear to be formed through cytochrome P450 3A4.
On the basis of the metabolism of bexarotene by cytochrome P450 3A4, concomitant ketoconazole, itraconazole, erythromycin and grapefruit juice could increase bexarotene plasma concentrations. Similarly, based on data that gemfibrozil increases bexarotene concentrations following oral bexarotene administration, concomitant gemfibrozil could increase bexarotene plasma concentrations. However, due to the low systemic exposure to bexarotene after low to moderately intense gel regimens (see Clinical Pharmacology), increases that occur are unlikely to be of sufficient magnitude to result in adverse effects.
No drug interaction data are available on concomitant administration of Targretin® gel and other CTCL therapies.
Renal Insufficiency
No formal studies have been conducted with Targretin® gel in patients with renal insufficiency. Urinary elimination of bexarotene and its known metabolites is a minor excretory pathway for bexarotene (<1% of an orally administered dose), but because renal insufficiency can result in significant protein binding changes, and bexarotene is >99% protein bound, pharmacokinetics may be altered in patients with renal insufficiency.
Hepatic Insufficiency
No specific studies have been conducted with Targretin® gel in patients with hepatic insufficiency. Because less than 1% of the dose of oral bexarotene is excreted in the urine unchanged and there isin vitro evidence of extensive hepatic contribution to bexarotene elimination, hepatic impairment would be expected to lead to greatly decreased clearance.
Protein Binding
Bexarotene is highly bound (>99%) to plasma proteins. The plasma proteins to which bexarotene binds have not been elucidated, and the ability of bexarotene to displace drugs bound to plasma proteins and the ability of drugs to displace bexarotene binding have not been studied.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to assess the carcinogenic potential of bexarotene have not been conducted. Bexarotene was not mutagenic to bacteria (Ames assay) or mammalian cells (mouse lymphoma assay). Bexarotene was not clastogenic in vivo (micronucleus test in mice). No formal fertility studies were conducted with bexarotene. Bexarotene caused testicular degeneration when oral doses of 1.5 mg/kg/day were given to dogs for 91 days.
Use in Nursing Mothers
It is not known whether bexarotene is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from bexarotene, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
Of the total patients with CTCL in clinical studies of Targretin® gel, 62% were under 65 years and 38% were 65 years or older. No overall differences in safety were observed between patients 65 years of age or older and younger patients, but greater sensitivity of some older individuals to Targretin® gel cannot be ruled out. Responses to Targretin® gel were observed across all age group decades, without preference for any individual age group decade.
-
ADVERSE REACTIONS
The safety of Targretin® gel has been assessed in clinical studies of 117 patients with CTCL who received Targretin® gel for up to 172 weeks. In the multicenter open label study, 50 patients with CTCL received Targretin® gel for up to 98 weeks. The mean duration of therapy for these 50 patients was 199 days. The most common adverse events reported with an incidence at the application site of at least 10% in patients with CTCL were rash, pruritus, skin disorder, and pain.
Adverse events leading to dose reduction or study drug discontinuation in at least two patients were rash, contact dermatitis, and pruritus.
Of the 49 patients (98%) who experienced any adverse event, most experienced events categorized as mild (9 patients, 18%) or moderate (27 patients, 54%). There were 12 patients (24%) who experienced at least one moderately severe adverse event. The most common moderately severe events were rash (7 patients, 14%) and pruritus (3 patients, 6%). Only one patient (2%) experienced a severe adverse event (rash).
In the patients with CTCL receiving Targretin® gel, adverse events reported regardless of relationship to study drug at an incidence of ≥5% are presented in Table 1.
A similar safety profile for Targretin® gel was demonstrated in the Phase I-II program. For the 67 patients enrolled in the Phase I-II program, the mean duration of treatment was 436 days (range 12-1203 days). As in the multicenter study, the most common adverse events regardless of relationship to study drug in the Phase I-II program were rash (78%), pain (40%), and pruritus (40%).
Table 1. Incidence of All Adverse Events* and Application Site Adverse Events with Incidence ≥5% for All Application Frequencies of Targretin® Gel in the Multicenter CTCL Study All Adverse Events Application Site Adverse Events COSTART 5
Body System/Preferred TermN = 50
n (%)N = 50
n (%)* Regardless of association with treatment
Includes Investigator terms such as:
1 Contact dermatitis, irritant contact dermatitis, irritant dermatitis
2 Pruritus, itching, itching of lesion
3 Erythema, scaling, irritation, redness, rash, dermatitis
4 Skin inflammation, excoriation, sticky or tacky sensation of skin; NOS = Not Otherwise Specified
Skin and Appendages
Contact Dermatitis1
7 (14)
4 (8)
Exfoliative Dermatitis
3 (6)
0
Pruritus2
18 (36)
9 (18)
Rash3
36 (72)
28 (56)
Maculopapular Rash
3 (6)
0
Skin Disorder (NOS)4
13 (26)
9 (18)
Sweating
3 (6)
0
Body as a Whole
Asthenia
3 (6)
0
Headache
7 (14)
0
Infection
9 (18)
0
Pain
15 (30)
9 (18)
Cardiovascular
Edema
5 (10)
0
Peripheral Edema
3 (6)
0
Hemic and Lymphatic
Leukopenia
3 (6)
0
Lymphadenopathy
3 (6)
0
WBC Abnormal
3 (6)
0
Metabolic and Nutritional
Hyperlipemia
5 (10)
0
Nervous
Paresthesia
3 (6)
3 (6)
Respiratory
Cough Increased
3 (6)
0
Pharyngitis
3 (6)
0
-
OVERDOSAGE
Systemic toxicity following acute overdosage with topical application of Targretin® gel is unlikely because of low systemic plasma levels observed with normal therapeutic doses. There is no specific antidote for overdosage.
There has been no experience with acute overdose of Targretin® gel in humans. Any overdose with Targretin® gel should be treated with supportive care for the signs and symptoms exhibited by the patient.
-
DOSAGE AND ADMINISTRATION
Targretin® gel should be initially applied once every other day for the first week. The application frequency should be increased at weekly intervals to once daily, then twice daily, then three times daily and finally four times daily according to individual lesion tolerance. Generally, patients were able to maintain a dosing frequency of two to four times per day. Most responses were seen at dosing frequencies of two times per day and higher. If application site toxicity occurs, the application frequency can be reduced. Should severe irritation occur, application of drug can be temporarily discontinued for a few days until the symptoms subside. See CONTRAINDICATIONS: Pregnancy: Category X.
Sufficient gel should be applied to cover the lesion with a generous coating. The gel should be allowed to dry before covering with clothing. Because unaffected skin may become irritated, application of the gel to normal skin surrounding the lesions should be avoided. In addition, do not apply the gel near mucosal surfaces of the body.
A response may be seen as soon as 4 weeks after initiation of therapy but most patients require longer application. With continued application, further benefit may be attained. The longest onset time for the first response among the responders was 392 days based on the Composite Assessment of Index Lesion Severity in the multicenter study. In clinical trials, Targretin® gel was applied for up to 172 weeks.
Targretin® gel should be continued as long as the patient is deriving benefit.
Occlusive dressings should not be used with Targretin® gel.
Targretin® gel is a topical therapy and is not intended for systemic use. Targretin® gel has not been studied in combination with other CTCL therapies.
-
HOW SUPPLIED
Targretin® gel is supplied in tubes containing 60 g (600 mg active bexarotene).
60 g tube ............................................................................................. NDC: 64365-504-01
Store at 25°C (77°F); with excursions permitted to 15°-30°C (59°-86°F) [see USP]. Avoid exposing to high temperatures and humidity after the tube is opened. Protect from light.
Manufactured for: Ligand Pharmaceuticals Incorporated
San Diego, CA 92121by: Bristol-Myers Squibb Company
Princeton, NJ 08543 USALigand Part #3000204 (Rev. 0101)
-
INGREDIENTS AND APPEARANCE
TARGRETIN
bexarotene gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 64365-504 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength bexarotene (UNII: A61RXM4375) (bexarotene - UNII:A61RXM4375) 10 mg in 1 g Inactive Ingredients Ingredient Name Strength dehydrated alcohol () polyethylene glycol 400 () hydroxypropyl cellulose () butylated hydroxytoluene () Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64365-504-01 60 g in 1 TUBE Labeler - Ligand Pharmaceuticals Inc.
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.