ECOZA- econazole nitrate aerosol, foam
ECOZA by
Drug Labeling and Warnings
ECOZA by is a Prescription medication manufactured, distributed, or labeled by Glenmark Therapeutics Inc.,USA, DPT Laboratories, Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ECOZA safely and effectively. See full prescribing information for ECOZA.
ECOZA® (econazole nitrate) topical foam
Initial U.S. Approval: 1982INDICATIONS AND USAGE
- Ecoza is an azole antifungal indicated for the treatment of interdigital tinea pedis caused by Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum in patients 12 years of age and older. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Topical foam, 1%. (3)
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Contents are flammable. Instruct the patient to avoid heat, flame, and/or smoking during and immediately following application. (5.1)
ADVERSE REACTIONS
The most common adverse reactions were application site reactions which occurred in less than 1% of subjects in both the Ecoza and vehicle arms. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Glenmark Therapeutics Inc., at 1-888-721-7115 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Flammability
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Warfarin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/ STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Flammability
Ecoza is flammable. Avoid heat, flame, and smoking during and immediately following application. Contents under pressure. Do not puncture and/or incinerate the containers. Do not expose containers to heat and/or store at temperatures above 120°F (49°C) even when empty. Do not store in direct sunlight.
-
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two double-blind, vehicle-controlled clinical trials, 495 subjects with interdigital tinea pedis applied Ecoza or vehicle once daily for approximately 28 days (246 subjects were exposed to Ecoza and 249 were exposed to vehicle). During clinical trials with Ecoza, the most common adverse reactions were application site reactions which occurred in less than 1% of subjects in both the Ecoza and vehicle arms.
-
7 DRUG INTERACTIONS
7.1 Warfarin
Concomitant administration of econazole and warfarin has resulted in enhancement of anticoagulant effect. Most cases reported product application with use under occlusion, genital application, or application to a large body surface area which may increase the systemic absorption of econazole nitrate. Monitoring of International Normalized Ratio (INR) and/or prothrombin time may be indicated especially for patients who apply econazole to large body surface areas, in the genital area, or under occlusion.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Ecoza use in pregnant women to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
In animal reproduction studies, econazole nitrate did not cause malformation in mice, rabbits and/or rats at oral doses 80 or 40 times the human dermal dose (see Data).
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Econazole nitrate did not cause malformation in mice, rabbits and/or rats. Fetotoxic or embryotoxic effects were observed in oral fertility studies in rats receiving 10 to 40 times the human dermal dose. Similar effects were observed in embryofetal and pre- and postnatal developmental studies in mice, rabbits and/or rats receiving oral doses 80 or 40 times the human dermal dose.
8.2 Lactation
Risk Summary
There is no information available on the presence of econazole nitrate in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production after topical application of Ecoza to women who are breastfeeding. It is not known whether econazole nitrate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when econazole nitrate is administered to a nursing woman. Following oral administration of econazole nitrate to lactating rats, econazole and/or metabolites were excreted in milk and were found in nursing pups.
The lack of clinical data during lactation precludes a clear determination of the risk Ecoza to an infant during lactation. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Ecoza and any potential adverse effects on the breastfed infant from Ecoza or from the underlying maternal condition.
8.4 Pediatric Use
Of the 173 subjects treated with Ecoza in the clinical trials, 2 subjects were 12-17 years old. In a pediatric maximal use trial, Ecoza was applied once daily to eighteen subjects aged 12 to 17 years with interdigital tinea pedis for 28 days [see Clinical Pharmacology (12.3)]. The safety findings for subjects 12 to 17 years were similar to those in adult population.
-
11 DESCRIPTION
Ecoza (econazole nitrate) topical foam, 1% contains the azole antifungal agent, econazole nitrate in an oil-in-water emulsion base. Each gram of Ecoza topical foam, 1% contains 10 mg of econazole nitrate, USP, in a white to off-white foam. Ecoza topical foam, 1% is alcohol (ethanol)‑ free and for topical use only.
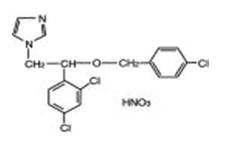
Chemically, econazole nitrate is 1-[2-{(4-chloro-phenyl)methoxy}-2-(2,4-dichlorophenyl)ethyl]‑ 1H-imidazole mononitrate. Econazole nitrate has the molecular formula C18H15Cl3N2O.HNO3 and a molecular weight of 444.70. Its molecular structure is as follows:
Ecoza (econazole nitrate) topical foam contains the following inactive ingredients: dimethicone, glycerin, polysorbate 20, povidone, propylene glycol, stearic acid, trolamine, purified water and butane as a propellant.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
The systemic absorption of Ecoza following topical application was studied in one clinical trial in adults and one clinical study in pediatric subjects.
In the adult trial, 19 subjects (male and female) with tinea pedis applied Ecoza once daily for 29 days. Subjects applied a mean daily amount of 2.4 g of Ecoza to soles, toes, interdigital spaces and tops of both feet up to the ankles. Blood samples were obtained on Day 29 at pre-dose and 1, 2, 4, 6, 8, and 12 hours after application. Results (mean ± SD) showed the time to reach peak plasma concentrations (Tmax) was 6.8 ± 5.1 h with maximum concentration (Cmax) of 417 ± 218 pg/mL. The area under the concentration time curve for the first 12 hours post application on Day 29 (AUC(0-12)) was 3440 ± 1920 pg-h/ml.
In the pediatric trial, 18 subjects (male and female ages 12 - 17) with interdigital tinea pedis and positive fungal cultures were treated with Ecoza once daily for 4 weeks. Subjects applied a mean daily amount of 3.2 g of Ecoza to soles, toes, interdigital spaces and tops of both feet up to the ankles. Blood samples were obtained on Day 28 at pre-dose and 7 h and 11 h post-dose. The mean ± SD econazole plasma concentration was 397 ± 289, 534 ± 745 and 575 ± 638 pg/mL at pre-dose and 7 h and 11 h post-dose, respectively.
Drug Interaction Studies
Ecoza is not expected to inhibit CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A4, or induce CYP1A2, 2B6, and 3A4.
12.4 Microbiology
Mechanism of Action
Econazole nitrate, an azole antifungal agent, inhibits fungal cytochrome P-450-mediated 14 alpha‑ lanosterol demethylase enzyme. This enzyme functions to convert lanosterol to ergosterol. The accumulation of 14 alpha-methyl sterols correlates with the subsequent loss of ergosterol in the fungal cell wall and may be responsible for the fungistatic activity of econazole. Mammalian cell demethylation is less sensitive to econazole inhibition.
Activity in vitro and in clinical infections
Econazole nitrate has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)]
Trichophyton rubrum
Epidermophyton floccosum
Trichophyton mentagrophytes
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
In two multi-center, randomized, double-blind, vehicle-controlled clinical trials a total of 505 subjects with interdigital tinea pedis were randomized 1:1 to Ecoza or vehicle; subjects applied the assigned medication once daily for 4 weeks. The severity of erythema, scaling, fissuring, maceration, vesiculation, and pruritus were graded using a 4-point scale (none, mild, moderate, severe). Subjects had KOH examination and fungal cultures taken to confirm eligibility. A total of 339 subjects with positive fungal cultures were evaluated for efficacy. Efficacy was evaluated on Day 43, 2 weeks post-treatment with treatment success being defined as complete cure (negative KOH and fungal culture and no evidence of clinical disease). The study population ranged in age from 12 to 71 years with 3 subjects less than 18 years of age at baseline. The subjects were 71% male and 52% Caucasian. Table 1 presents the efficacy results for each trial.
Table 1: Efficacy Results at Two Weeks Post-treatment (Day 43) Complete Cure, Effective Treatment and Mycological Cure Study 1 Study 2 Ecoza
N = 82
n(%)Foam Vehicle
N = 83
n(%)Ecoza
N = 91
n(%)Foam Vehicle
N = 83
n(%)Complete curea
19 (23.2%)
2 (2.4%)
23 (25.3%)
4 (4.8%)
Effective treatmentb
40 (48.8%)
9 (10.8%)
44 (48.4%)
9 (10.8%)
Mycological curec
56 (68.3%)
13 (15.7%)
61 (67.0%)
15 (18.1%)
- a Mycological cure and an absence of clinical signs and symptoms (erythema, scaling, fissuring, maceration, vesiculation, or pruritus).
- b Mycological cure and no or mild erythema and/or scaling with all other signs and symptoms absent.
- c Negative KOH and fungal culture.
-
16 HOW SUPPLIED/ STORAGE AND HANDLING
Ecoza topical foam, 1% is white to off-white foam supplied in 70 g (NDC: 72657-0200-70) aluminum pressurized canister.
Store at controlled room temperature 20°C to 25°C (68°F to 77°F) with excursions permitted between 15°C and 30°C (59°F and 86°F). Do not refrigerate or freeze.
Ecoza topical foam is flammable. Avoid heat, flame, and smoking during and immediately following application.
Contents under pressure. Do not puncture and/or incinerate the containers.
Do not expose containers to heat and/or store at temperatures above 120°F (49°C) even when empty.
Do not store in direct sunlight.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
The patient should be instructed as follows:
- Inform patients that Ecoza is for topical use only. Ecoza is not intended for oral, intravaginal, or ophthalmic use.
- Ecoza is flammable; avoid heat, flame, and smoking during and immediately following application.
- If a reaction suggesting sensitivity or chemical irritation develops with the use of Ecoza use of the medication should be discontinued.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Patient Information
ECOZA® (ee-ko-zah)
(econazole nitrate) topical foam, 1%Important information: Ecoza topical foam is for use on skin only. Do not use Ecoza topical foam in your eyes or vagina.
What is Ecoza topical foam?
Ecoza topical foam is a prescription medicine used on the skin (topical) to treat athlete's foot that is between the toes (interdigital tinea pedis) in people 12 years of age and older.-
What should I tell my doctor before using Ecoza topical foam?
Before using Ecoza topical foam, tell your doctor about all of your medical conditions, including if you:
- 1. are pregnant or plan to become pregnant. It is not known if Ecoza topical foam will harm your unborn baby.
- 2. are breastfeeding or plan to breastfeed. It is not known if Ecoza topical foam passes into your breast milk.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.-
How should I use Ecoza topical foam?
See the detailed Instructions for Use for information about how to use Ecoza topical foam.
- 1. Use Ecoza topical foam exactly as your doctor tells you to use it.
- 2. Apply Ecoza topical foam to the affected skin areas of your feet 1 time a day for 4 weeks.
- 3. If Ecoza topical foam gets in or near your eyes, rinse them well with water.
- 4. Wash your hands after you apply Ecoza topical foam.
What should I avoid while using Ecoza topical foam?
- Ecoza topical foam is flammable. Avoid heat, flame and smoking while applying and right after you apply Ecoza topical foam to your skin.
What are the possible side effects of Ecoza topical foam?
Ecoza topical foam may cause skin reactions at the treatment site. Tell your doctor if you have any skin reactions on the areas of your skin treated with Ecoza topical foam.
These are not all the possible side effects of Ecoza topical foam.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store Ecoza topical foam?
- Store Ecoza topical foam at room temperature, between 68°F to 77°F (20°C to 25°C).
- Do not refrigerate or freeze Ecoza topical foam.
- Do not store Ecoza topical foam in direct sunlight.
- Ecoza topical foam is flammable. Keep the Ecoza topical foam canister away from heat and temperatures above 120°F (49°C), even if the canister is empty.
- Do not puncture or burn the Ecoza topical foam canister.
Keep Ecoza topical foam and all medicines out of the reach of children.General information about the safe and effective use of Ecoza topical foam
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your doctor or pharmacist for information about Ecoza topical foam that is written for health professionals. Do not use Ecoza topical foam for a condition for which it was not prescribed. Do not give Ecoza topical foam to other people, even if they have the same symptoms that you have. It may harm them.What are the ingredients in Ecoza topical foam?
Active ingredient: econazole nitrate
Inactive Ingredients: dimethicone, glycerin, polysorbate 20, povidone, propylene glycol, stearic acid, trolamine, purified water and butane as a propellant.Marketed by:
Glenmark Therapeutics Inc.
Mahwah, NJ 07430
For more information call Glenmark Therapeutics Inc., USA at 1-888-721-7115.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: 03/2019
-
What should I tell my doctor before using Ecoza topical foam?
-
Instructions for Use
ECOZA® (ee-ko-zah)
(econazole nitrate) topical foam, 1%Important information: Ecoza® topical foam is for use on skin only. Do not use Ecoza topical foam in your eyes or vagina.
Parts of Ecoza topical foam Canister. (See Figure A)
How to apply Ecoza topical foam:
Step 1:
Before you apply Ecoza topical foam, shake the Ecoza topical foam canister for about 5 seconds.
Step 2:
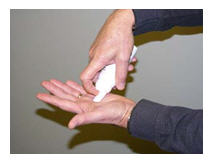
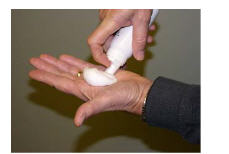
Remove the cap and turn the Ecoza topical foam canister upside down over the palm of your hand.
Step 3:
Press down firmly on the actuator until there is a small amount of foam about the size of a golf ball in the palm of your hand. (See Figures B and C)
Step 4:
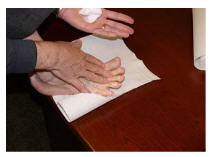
Use your finger-tips to scoop up small amounts of Ecoza topical foam and apply to the affected skin areas on your feet. Gently rub the foam into the skin. (See Figure D)
Step 5:
You should apply Ecoza topical foam to your toes, to the spaces between your toes, and to the surrounding areas 1 time a day for 4 weeks or as prescribed by your doctor.
Step 6:
Replace the cap. Wash your hands after applying Ecoza topical foam.
How should I store Ecoza topical foam?
- Store Ecoza topical foam at room temperature, between 68°F to 77°F (20°C to 25°C).
- Do not refrigerate or freeze Ecoza topical foam.
- Do not store Ecoza topical foam in direct sunlight.
- Ecoza topical foam is flammable. Keep the Ecoza topical foam canister away from heat and temperatures above 120°F (49°C), even if the canister is empty.
- Do not puncture or burn the Ecoza topical foam canister.
Keep Ecoza topical foam and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Marketed by
Glenmark Therapeutics Inc.
Mahwah, NJ 07430
Issued: 03/2019
-
PRINCIPAL DISPLAY PANEL - 70 g Can Label
NDC: 72657-200-70
ecoza®
(econazole nitrate)
topical foam, 1%For Topical Use Only
Not for ophthalmic, oral or intravaginal use.Keep Out of Reach of Children
Rx Only
Net Wt 70g -
PRINCIPAL DISPLAY PANEL - 70 g Canister Carton
NDC: 72657-200-70
ecoza®
(econazole nitrate)
topical foam, 1%For Topical Use Only
Not for ophthalmic, oral
or intravaginal use.Keep Out of Reach of Children
Rx Only
Net Wt 70g -
INGREDIENTS AND APPEARANCE
ECOZA
econazole nitrate aerosol, foamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72657-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ECONAZOLE NITRATE (UNII: H438WYN10E) (ECONAZOLE - UNII:6Z1Y2V4A7M) ECONAZOLE NITRATE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 20 (UNII: 7T1F30V5YH) POVIDONE K30 (UNII: U725QWY32X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) BUTANE (UNII: 6LV4FOR43R) Product Characteristics Color WHITE (white to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72657-200-70 1 in 1 CARTON 10/25/2013 1 70 g in 1 CAN; Type 0: Not a Combination Product 2 NDC: 72657-200-99 1 in 1 CARTON 10/25/2013 2 10 g in 1 CAN; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205175 10/25/2013 Labeler - Glenmark Therapeutics Inc.,USA (969085666) Registrant - Glenmark Therapeutics Inc.,USA (969085666) Establishment Name Address ID/FEI Business Operations DPT Laboratories, Ltd. 832224526 ANALYSIS(72657-200) , LABEL(72657-200) , MANUFACTURE(72657-200) , PACK(72657-200)
Trademark Results [ECOZA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ECOZA 97432935 not registered Live/Pending |
Resilia Pharmaceuticals, Inc. 2022-05-27 |
 ECOZA 87019005 5162832 Live/Registered |
GLENMARK THERAPEUTICS INC., USA 2016-04-29 |
 ECOZA 86092555 4822727 Live/Registered |
GLENMARK THERAPEUTICS INC., USA 2013-10-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.



 s
s
