BEXSERO- neisseria meningitidis serogroup b nhba fusion protein antigen, neisseria meningitidis serogroup b fhbp fusion protein antigen and neisseria meningitidis serogroup b nada protein antigen injection, suspension
Bexsero by
Drug Labeling and Warnings
Bexsero by is a Other medication manufactured, distributed, or labeled by GlaxoSmithKline Biologicals SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BEXSERO safely and effectively. See full prescribing information for BEXSERO.
BEXSERO (Meningococcal Group B Vaccine) suspension, for intramuscular injection
Initial U.S. Approval: 2015INDICATIONS AND USAGE
BEXSERO is a vaccine indicated for active immunization to prevent invasive disease caused by Neisseria meningitidis serogroup B. BEXSERO is approved for use in individuals aged 10 through 25 years. (1)
Approval of BEXSERO is based on demonstration of immune response, as measured by serum bactericidal activity against three serogroup B strains representative of prevalent strains in the United States. The effectiveness of BEXSERO against diverse serogroup B strains has not been confirmed. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Suspension for intramuscular injection in 0.5-mL single-dose prefilled syringes. (3)
CONTRAINDICATIONS
Hypersensitivity, including severe allergic reaction, to any component of the vaccine, or after a previous dose of BEXSERO. (4)
WARNINGS AND PRECAUTIONS
The tip caps of the prefilled syringes contain natural rubber latex which may cause allergic reactions. (5.3)
ADVERSE REACTIONS
The most common solicited adverse reactions observed in clinical trials were pain at the injection site (≥83%), myalgia (≥48%), erythema (≥45%), fatigue (≥35%), headache (≥33%), induration (≥28%), nausea (≥18%), and arthralgia (≥13%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
2.2 Administration
2.3 Use of BEXSERO with Other Meningococcal Group B Vaccines
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Preventing and Managing Allergic Reactions
5.2 Syncope
5.3 Latex
5.4 Limitation of Vaccine Effectiveness
5.5 Altered Immunocompetence
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Additional Pre-licensure Safety Experience
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Immunogenicity
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
BEXSERO is a vaccine indicated for active immunization to prevent invasive disease caused by Neisseria meningitidis serogroup B. BEXSERO is approved for use in individuals aged 10 through 25 years.
Approval of BEXSERO is based on demonstration of immune response, as measured by serum bactericidal activity against three serogroup B strains representative of prevalent strains in the United States. The effectiveness of BEXSERO against diverse serogroup B strains has not been confirmed.
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use only.
2.2 Administration
Shake the syringe immediately before use to form a homogeneous suspension. Do not use the vaccine if it cannot be resuspended. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter or discoloration is found.
Administer BEXSERO as a 0.5-mL intramuscular injection into the deltoid muscle of the upper arm.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Hypersensitivity, including severe allergic reaction, to any component of the vaccine, or after a previous dose of BEXSERO [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Preventing and Managing Allergic Reactions
Appropriate observation and medical treatment should always be readily available in case of an anaphylactic reaction following the administration of the vaccine.
5.2 Syncope
Syncope (fainting) can occur in association with administration of BEXSERO. Ensure procedures are in place to avoid injury from falling associated with syncope.
5.3 Latex
The tip caps of the prefilled syringes contain natural rubber latex which may cause allergic reactions.
5.4 Limitation of Vaccine Effectiveness
BEXSERO may not protect all vaccine recipients. BEXSERO may not provide protection against all meningococcal serogroup B strains [see Clinical Pharmacology (12.1)].
5.5 Altered Immunocompetence
Some individuals with altered immunocompetence may have reduced immune responses to BEXSERO.
Complement Deficiency
Persons with certain complement deficiencies and persons receiving treatment that inhibits terminal complement activation (for example, eculizumab) are at increased risk for invasive disease caused by N. meningitidis serogroup B even if they develop antibodies following vaccination with BEXSERO. [See Clinical Pharmacology (12.1).]
-
6 ADVERSE REACTIONS
The most common solicited adverse reactions observed in clinical trials were pain at the injection site (≥83%), myalgia (≥48%), erythema (≥45%), fatigue (≥35%), headache (≥33%), induration (≥28%), nausea (≥18%), and arthralgia (≥13%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
In 4 clinical trials, 3,058 individuals aged 10 through 25 years received at least one dose of BEXSERO, 1,436 participants received only BEXSERO, 2,089 received only placebo or a control vaccine, and 1,622 participants received a mixed regimen (placebo or control vaccine and BEXSERO).
In a randomized controlled study1 conducted in U.S. and Poland, 120 participants aged 10 through 25 years received at least 1 dose of BEXSERO, including 112 participants who received 2 doses of BEXSERO 2 months apart; 97 participants received saline placebo followed by MENVEO [Meningococcal (Groups A, C, Y, and W-135) Oligosaccharide Diphtheria CRM197 Conjugate Vaccine]. Across groups, median age was 13 years, males comprised 49%, and 60% were white, 34% were Hispanic, 4% were black, <1% were Asian, and 2% were other.
In a second randomized controlled study2 conducted in Chile, all subjects (N = 1,622) aged 11 through 17 years received at least 1 dose of BEXSERO. This study included a subset of 810 subjects who received 2 doses of BEXSERO 1 or 2 months apart. A control group of 128 subjects received at least 1 dose of placebo containing aluminum hydroxide. A subgroup of 128 subjects received 2 doses of BEXSERO 6 months apart. In this study, median age was 14 years, males comprised 44%, and 99% were Hispanic.
In a third randomized controlled study3 conducted in the United Kingdom (U.K.), 974 university students aged 18 through 24 years received at least 1 dose of BEXSERO, including 932 subjects who received 2 doses of BEXSERO 1 month apart. Comparator groups received 1 dose of MENVEO followed by 1 dose of placebo containing aluminum hydroxide (n = 956) or 2 doses of IXIARO (Japanese Encephalitis Vaccine, Inactivated, Adsorbed) (n = 947). Across groups, median age was 20 years, males comprised 46%, and 88% were white, 5% were Asian, 2% were black, <1% were Hispanic, and 4% were other.
In an uncontrolled study4 conducted in Canada and Australia, 342 participants aged 11 through 17 years received at least 1 dose of BEXSERO, including 338 participants who received 2 doses of BEXSERO 1 month apart. The median age was 13 years, males comprised 55%, and 80% were white, 10% were Asian, 4% were Native American/Alaskan, and 4% were other.
Local and systemic reactogenicity data were solicited from all participants in the studies conducted in Chile, U.S./Poland, Canada/Australia, and in a subset of participants in the U.K. study. Reports of unsolicited adverse events occurring within the first 7 days after each vaccination were collected in all studies. In the U.S./Poland study, reports of unsolicited adverse events were collected up to 1 month after the second vaccination.
Reports of all serious adverse events, medically attended adverse events, and adverse events leading to premature withdrawal were collected throughout the study period for the studies conducted in Chile (12 months), U.K. (12 months), U.S./Poland (8 months), and Canada/Australia (2 months).
Solicited Adverse Reactions
The reported rates of local and systemic reactions among participants aged 10 through 25 years following each dose of BEXSERO administered 2 months apart or control in the U.S./Polish study1 are presented in Table 1.
Table 1. Percentage of U.S. and Polish Participants Aged 10 through 25 Years Reporting Solicited Local and Systemic Adverse Reactions within 7 Days after BEXSERO or Control, by Dose Clinicaltrials.gov Identifier NCT01272180.
a Erythema and induration: Any (≥1 mm). Pain and systemic reactions: Mild (transient with no limitation in normal daily activity); Moderate (some limitation in normal daily activity); Severe (unable to perform normal daily activity).
b Administered 2 months after Dose 1.Solicited Reactiona
Dose 1
Dose 2b
BEXSERO
Placebo
(Saline)
BEXSERO
MENVEO
n = 110-114
n = 94-96
n = 107-109
n = 90-92
Local Adverse Reactions
Pain
Any
90
27
83
43
Mild
27
20
18
26
Moderate
44
5
37
9
Severe
20
2
29
8
Erythema
Any
50
13
45
26
1-25 mm
41
11
36
13
>25-50 mm
6
1
5
6
>50-100 mm
3
0
5
4
>100 mm
0
0
0
2
Induration
Any
32
10
28
23
1-25 mm
24
9
22
16
>25-50 mm
7
0
4
0
>50-100 mm
1
1
2
4
>100 mm
0
0
0
2
Systemic Adverse Reactions
Fatigue
Any
37
22
35
20
Mild
19
17
18
11
Moderate
14
5
10
7
Severe
4
0
6
2
Nausea
Any
19
4
18
4
Mild
12
3
10
3
Moderate
4
1
5
1
Severe
4
0
4
0
Myalgia
Any
49
26
48
25
Mild
21
20
16
14
Moderate
16
5
19
7
Severe
12
1
13
4
Arthralgia
Any
13
4
16
4
Mild
9
3
8
2
Moderate
3
1
6
2
Severe
2
0
2
0
Headache
Any
33
20
34
23
Mild
19
15
21
8
Moderate
9
4
6
12
Severe
4
1
6
3
Fever
≥38°C
1
1
5
0
38.0-38.9°C
1
1
4
0
39.0-39.9°C
0
0
1
0
≥40°C
0
0
0
0
Solicited adverse reaction rates were similar among participants aged 11 through 24 years who received BEXSERO in the other 3 clinical studies,2,3,4 except for severe myalgia which was reported by 3% to 7% of subjects. Severe pain was reported by 8% of university students in the U.K.3
Non-serious Adverse Reactions
In the 3 controlled studies1,2,3 (BEXSERO n = 2,221, control n = 2,204), non-serious unsolicited adverse events that occurred within 7 days of any dose were reported by 439 (20%) participants receiving BEXSERO and 197 (9%) control recipients. Unsolicited adverse reactions that were reported among at least 2% of participants and were more frequently reported in participants receiving BEXSERO than in control recipients were injection site pain, headache, injection site induration unresolved within 7 days, and nasopharyngitis.
Serious Adverse Events
Overall, in clinical studies, among 3,058 participants aged 10 through 25 years who received at least 1 dose of BEXSERO, 66 (2.1%) participants reported serious adverse events at any time during the study. In the 3 controlled studies1,2,3 (BEXSERO n = 2,716, control n = 2,078), serious adverse events within 30 days after any dose were reported in 23 (0.8%) participants receiving BEXSERO and 10 (0.5%) control recipients.
6.2 Additional Pre-licensure Safety Experience
In response to outbreaks of serogroup B meningococcal disease at 2 universities in the U.S., BEXSERO was administered as a 2-dose series at least 1 month apart. Information on serious adverse events was collected for a period of 30 days after each dose from 15,351 individuals aged 16 through 65 years who received at least 1 dose. Overall 50 individuals (0.3%) reported serious adverse events, including one reaction considered related to vaccination, a case of anaphylaxis within 30 minutes following vaccination.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of BEXSERO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
General Disorders and Administration Site Conditions
Injection site reactions (including extensive swelling of the vaccinated limb, blisters at or around the injection site, and injection site nodule which may persist for more than 1 month).
Immune System Disorders
Allergic reactions (including anaphylactic reactions), rash, eye swelling.
Nervous System Disorders
Syncope, vasovagal responses to injection.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
There are no adequate and well-controlled studies of BEXSERO in pregnant women in the U.S. Available human data on BEXSERO administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
A developmental toxicity study was performed in female rabbits administered BEXSERO prior to mating and during gestation. The dose was 0.5 mL at each occasion (a single human dose is 0.5 mL). This study revealed no adverse effects on fetal or pre-weaning development due to BEXSERO (see Data).
Data
Animal Data: In a developmental toxicity study, female rabbits were administered BEXSERO by intramuscular injection on Days 29, 15, and 1 prior to mating and on Gestation Days 7 and 20. The total dose was 0.5 mL at each occasion (a single human dose is 0.5 mL). No adverse effects on pre-weaning development up to Postnatal Day 29 were observed. There were no fetal malformations or variations observed.
8.2 Lactation
Risk Summary
It is not known whether the vaccine components of BEXSERO are excreted in human milk. Available data are not sufficient to assess the effects of BEXSERO on the breastfed infant or on milk production/excretion. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for BEXSERO and any potential adverse effects on the breastfed child from BEXSERO or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
-
11 DESCRIPTION
BEXSERO (Meningococcal Group B Vaccine) is a sterile, white, opalescent, suspension for intramuscular injection. Each 0.5-mL dose of BEXSERO is formulated to contain 50 micrograms each of recombinant proteins Neisserial adhesin A (NadA), Neisserial Heparin Binding Antigen (NHBA), and factor H binding protein (fHbp), 25 micrograms of Outer Membrane Vesicles (OMV), 1.5 mg aluminum hydroxide (0.519 mg of Al3+), 3.125 mg sodium chloride, 0.776 mg histidine, and 10 mg sucrose at pH 6.4 – 6.7.
The NadA component is a fragment of the full-length protein derived from N. meningitidis strain 2996 (peptide 8 variant 2/3)5. The NHBA component is a recombinant fusion protein comprised of NHBA (peptide 2)5 and accessory protein 953 derived from N. meningitidis strains NZ98/254 and 2996, respectively. The fHbp component is a recombinant fusion protein comprised of fHbp (variant 1.1)5 and the accessory protein 936 derived from N. meningitidis strains MC58 and 2996, respectively. These 3 recombinant proteins are individually produced in Escherichia coli and purified through a series of column chromatography steps. The OMV antigenic component is produced by fermentation of N. meningitidis strain NZ98/254 (expressing outer membrane protein PorA serosubtype P1.4)6, followed by inactivation of the bacteria by deoxycholate, which also mediates vesicle formation. The antigens are adsorbed onto aluminum hydroxide.
Each dose contains less than 0.01 micrograms kanamycin (by calculation).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Protection against invasive meningococcal disease is conferred mainly by complement-mediated antibody-dependent killing of N. meningitidis. The effectiveness of BEXSERO was assessed by measuring serum bactericidal activity using human complement (hSBA).
NHBA, NadA, fHbp, and PorA are proteins found on the surface of meningococci and contribute to the ability of the bacterium to cause disease. Vaccination with BEXSERO leads to the production of antibodies directed against NHBA, NadA, fHbp, and PorA P1.4 (present in OMV). The susceptibility of serogroup B meningococci to complement-mediated antibody-dependent killing following vaccination with BEXSERO is dependent on both the antigenic similarity of the bacterial and vaccine antigens, as well as the amount of antigen expressed on the surface of the invading meningococci.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
BEXSERO has not been evaluated for carcinogenic or mutagenic potential or impairment of male fertility in animals. Vaccination of female rabbits with BEXSERO had no effect on fertility. [See Use in Specific Populations (8.1).]
-
14 CLINICAL STUDIES
The immunogenicity of BEXSERO following 2 doses was evaluated in individuals aged 11 through 24 years. Serum bactericidal antibodies were measured with hSBA assays using 3 strains selected to measure responses to one of 3 vaccine antigens, either fHbp, NadA, or PorA P1.4, prevalent among strains in the U.S. A suitable strain for assessing bactericidal activity of NHBA-specific antibodies was not available. Studies assessed the proportion of subjects who achieved a 4-fold or greater increase in hSBA titer for each of the 3 strains, and the proportion of subjects with a titer greater than or equal to the lower limit of quantitation (LLOQ) of the assay for all 3 strains (composite response). The LLOQ was defined as the lowest amount of the antibody in a sample that can be reliably quantified. Available data showed that baseline antibody titers across populations vary.
14.1 Immunogenicity
In a clinical trial conducted in Canada and Australia, adolescents aged 11 through 17 years received 2 doses of BEXSERO 1 month apart. The hSBA responses 1 month after the second dose are shown in Table 2.
Table 2. Bactericidal Antibody Response Rates following 2 Doses of BEXSERO Administered 1 Month Apart to Canadian and Australian Adolescentsa NCT 01423084.
Abbreviations: CI = Confidence interval; hSBA = Serum bactericidal activity measured using human complement; LLOQ = Lower limit of quantitation.
a Evaluable Immunogenicity Population (aged 11 through 17 years).
b ≥4-fold hSBA response is defined as: a post-vaccination hSBA ≥1:16 for participants with pre-vaccination hSBA <1:4, a post-vaccination titer at least 4-fold the LLOQ for participants with pre-vaccination hSBA ≥1:4 but < LLOQ, and a post-vaccination 4-fold rise for participants with pre-vaccination hSBA ≥LLOQ.
c LLOQ = 1:16 for H44/76; 1:16 for 5/99; 1:8 for NZ98/254.
d Composite hSBA Response means hSBA ≥LLOQ for all 3 indicator Meningococcal B strains.≥4-Fold hSBA Response 1 Month Post Dose 2b,c
Strain (Antigen)
n
%
95% CI
H44/76 (fHbp)
298
98
95, 99
5/99 (NadA)
299
99
98, 100
NZ98/254 (PorA P1.4)
298
39
33, 44
Composite hSBA Responsec,d
Time Point
n
%
95% CI
Baseline (pre-vaccination)
299
0
—
1 Month Post Dose 2
298
63
57, 68
In a randomized, controlled clinical trial conducted in the U.K. among university students aged 18 through 24 years, hSBA responses in a subset of participants who received BEXSERO were measured 1 month and 11 months after the second dose (Table 3).
Table 3. Bactericidal Antibody Response Rates following 2 Doses of BEXSERO Administered 1 Month Apart to University Students in the U.K.a NCT 01214850.
Abbreviations: CI = Confidence interval; hSBA = Serum bactericidal activity measured using human complement; LLOQ = Lower limit of quantitation.
a Evaluable Immunogenicity Population (aged 18 through 24 years).
b ≥4-fold hSBA response is defined as: a post-vaccination hSBA ≥1:16 for participants with pre-vaccination hSBA <1:4, a post-vaccination titer at least 4-fold the LLOQ for participants with pre-vaccination hSBA ≥1:4 but <LLOQ, and a post-vaccination 4-fold rise for participants with pre-vaccination hSBA ≥LLOQ.
c LLOQ = 1:16 for H44/76; 1:8 for 5/99; 1:16 for NZ98/254.
d Composite hSBA Response means hSBA ≥LLOQ for all 3 indicator Meningococcal B strains.≥4-Fold hSBA Response 1 Month Post Dose 2b,c
Strain (Antigen)
n
%
95% CI
H44/76 (fHbp)
148
78
71, 85
5/99 (NadA)
148
94
89, 97
NZ98/254 (PorA P1.4)
147
67
58, 74
Composite hSBA Responsec,d
Time Point
n
%
95% CI
Baseline (pre-vaccination)
186
24
18,30
1 Month Post Dose 2
147
88
82,93
11 Months Post Dose 2
136
66
58,72
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
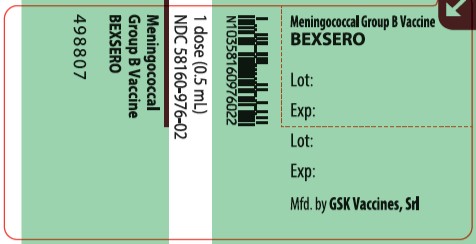
BEXSERO is supplied as a 0.5-mL suspension in a glass prefilled syringe (packaged without needles). The tip caps of the prefilled syringes contain natural rubber latex; the plungers are not made with natural rubber latex.
Table 4. Product Presentations for BEXSERO
Presentation
Carton NDC Number
Components
Pre-filled syringe
Carton of 1 syringe
58160-976-06
0.5-mL single-dose prefilled syringe
NDC: 58160-976-02
Carton of 10 syringes
58160-976-20
0.5-mL single-dose prefilled syringe
NDC: 58160-976-02
-
17 PATIENT COUNSELING INFORMATION
Give the patient, parent, or guardian the Vaccine Information Statements, which are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to immunization. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
Inform patients, parents, or guardians about:
- The importance of completing the immunization series.
- Reporting any adverse reactions to their healthcare provider.
BEXSERO and MENVEO are trademarks owned by or licensed to the GSK group of companies.
The other brand listed is a trademark owned by or licensed to its owner and is not owned by or licensed to the GSK group of companies. The maker of this brand is not affiliated with and does not endorse the GSK group of companies or its products.
Manufactured by GSK Vaccines, Srl
Bellaria-Rosia 53018, Sovicille (SI), Italy
U.S. License No. 1617
Distributed by GlaxoSmithKline
Research Triangle Park, NC 27709
©2019 GSK group of companies or its licensor.
BXS:5PI
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC: 58160-976-20
BEXSERO
Meningococcal Group B Vaccine
Rx Only
For Use from 10 through 25 Years of Age
10 Single-Dose Prefilled Syringes each containing one 0.5-mL dose
GlaxoSmithKline
Made in Italy
©2019 GSK group of companies or its licensor.
- 498805 Rev. 9/19
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BEXSERO
neisseria meningitidis serogroup b nhba fusion protein antigen, neisseria meningitidis serogroup b fhbp fusion protein antigen and neisseria meningitidis serogroup b nada protein antigen injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 58160-976 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEISSERIA MENINGITIDIS GROUP B NHBA FUSION PROTEIN ANTIGEN (UNII: 28E911Y7AE) (NEISSERIA MENINGITIDIS GROUP B NHBA FUSION PROTEIN ANTIGEN - UNII:28E911Y7AE) NEISSERIA MENINGITIDIS GROUP B NHBA FUSION PROTEIN ANTIGEN 50 ug in 0.5 mL NEISSERIA MENINGITIDIS GROUP B FHBP FUSION PROTEIN ANTIGEN (UNII: 25DB599G64) (NEISSERIA MENINGITIDIS GROUP B FHBP FUSION PROTEIN ANTIGEN - UNII:25DB599G64) NEISSERIA MENINGITIDIS GROUP B FHBP FUSION PROTEIN ANTIGEN 50 ug in 0.5 mL NEISSERIA MENINGITIDIS GROUP B NADA PROTEIN ANTIGEN (UNII: 1S25R442RS) (NEISSERIA MENINGITIDIS GROUP B NADA PROTEIN ANTIGEN - UNII:1S25R442RS) NEISSERIA MENINGITIDIS GROUP B NADA PROTEIN ANTIGEN 50 ug in 0.5 mL NEISSERIA MENINGITIDIS GROUP B STRAIN NZ98/254 OUTER MEMBRANE VESICLE (UNII: 91523M4S24) (NEISSERIA MENINGITIDIS GROUP B STRAIN NZ98/254 OUTER MEMBRANE VESICLE - UNII:91523M4S24) NEISSERIA MENINGITIDIS GROUP B STRAIN NZ98/254 OUTER MEMBRANE VESICLE 25 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM CHLORIDE (UNII: 451W47IQ8X) HISTIDINE (UNII: 4QD397987E) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58160-976-06 1 in 1 CARTON 1 NDC: 58160-976-02 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 58160-976-20 10 in 1 CARTON 2 NDC: 58160-976-02 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125546 12/01/2016 Labeler - GlaxoSmithKline Biologicals SA (372748392)
Trademark Results [Bexsero]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BEXSERO 79104116 4130376 Live/Registered |
Glaxosmithkline Biologicals S.A. 2011-09-22 |
 BEXSERO 77896927 3821791 Live/Registered |
GLAXOSMITHKLINE BIOLOGICALS S.A. 2009-12-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.