pipracil- Piperacillin sodium injection, powder, lyophilized, for solution
Drug Labeling and Warnings
Drug Details [pdf]
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
PIPRACIL, sterile piperacillin sodium, is a semisynthetic broad-spectrum penicillin for parenteral use derived from D(-)-α-aminobenzylpenicillin. The chemical name of piperacillin sodium is sodium (2S,5R,6R)-6-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)-2-phenylacetamido]-3,-3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate. The chemical formula is C23H26N5NaO7S, and the molecular weight is 539.54. Its structural formula is:
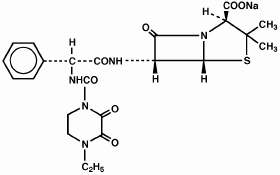
Piperacillin sodium powder is a white to off-white solid having the characteristic appearance of products prepared by freeze-drying. It is freely soluble in water and in alcohol. The pH of an aqueous solution containing 400 milligrams per milliliter ranges from 5.5 to 7.5. One g contains 1.85 mEq (42.5 mg) of sodium (Na+).
-
CLINICAL PHARMACOLOGY
Intravenous Administration:
In healthy adult volunteers, mean serum piperacillin concentrations immediately after a two‑to three‑minute intravenous injection of 2, 4, or 6 g were 305, 412, and 775 μg/mL, respectively. Serum concentrations lack dose proportionality.
PIPERACILLIN SERUM CONCENTRATIONS IN ADULTS (μg/mL) AFTER A TWO- TO THREE-MINUTE I.V. INJECTION DOSE 0 10 min 20 min 30 min 1 h 1.5 h 2 h 3 h 4 h 6 h 8 h 2 305
(159-
615)202
(164-
225)156
(52-
165)67
(41-
88)40
(25-
57)24
(18-
31)20
(14-
24)8
(3-
11)3
(2-
4)2
(≤0.6-
3)—
4 412
(389-
484)344
(315-
379)295
(269-
330)117
(98-
138)93
(78-
110)60
(50-
67)36
(26-
51)20
(17-
24)8
(7-
11)4
(3.7-
4.1)0.9
(0.7-
1)6 775
(695-
849)609
(530-
670)563
(492-
630)325
(292-
363)208
(180-
239)138
(115-
175)90
(71-
113)38
(29-
53)33
(25-
44)8
(3-
19)3.2
(<2-
6)PIPERACILLIN SERUM CONCENTRATIONS IN ADULTS (μg/mL) AFTER A 30-MINUTE I.V. INFUSION DOSE 0 5 min 10 min 15 min 30 min 45 min 1 h 1.5 h 2 h 4 h 6 h 7.5 h 4 244
(155-
298)215
(169-
247)186
(140-
209)177
(142-
213)141
(122-156)146
(110-
265)105
(85-
133)72
(53-
105)53
(36-
69)15
(6-
24)4
(1-
9)2
(0.5-
3)6 353
(324-
371)298
(242-
339)298
(232-
331)272
(219-314)229
(185-
249)180
(144-
209)149
(117-
171)104
(89-
113)73
(66-
94)22
(12-
39)16
(5-
49)—
—A 30-minute infusion of 6 g every 6 h gave, on the fourth day, a mean peak serum concentration of 420 μg/mL.
Intramuscular Administration:
PIPRACIL is rapidly absorbed after intramuscular injection. In healthy volunteers, the mean peak serum concentration occurs approximately 30 minutes after a single dose of 2 g and is about 36 μg/mL. The oral administration of 1 g probenecid before injection produces an increase in piperacillin peak serum level of about 30%. The area under the curve (AUC) is increased by approximately 60%.
Pharmacokinetics:
PIPRACIL is not absorbed when given orally. Peak serum concentrations are attained approximately 30 minutes after intramuscular injections and immediately after completion of intravenous injection or infusion. The serum half-life in healthy volunteers ranges from 36 minutes to one hour and 12 minutes. The mean elimination half-life of PIPRACIL in healthy adult volunteers is 54 minutes following administration of 2 g and 63 minutes following 6 g. As with other penicillins, PIPRACIL is eliminated primarily by glomerular filtration and tubular secretion; it is excreted rapidly as unchanged drug in high concentrations in the urine. Approximately 60% to 80% of the administered dose is excreted in the urine in the first 24 hours. Piperacillin urine concentrations, determined by microbioassay, are as high as 14,100 μg/mL following a 6-g intravenous dose and 8,500 μg/mL following a 4-g intravenous dose. These urine drug concentrations remain well above 1,000 μg/mL throughout the dosing interval.
Distribution:
PIPRACIL binding to human serum proteins is 16%. The drug is widely distributed in human tissues and body fluids, including bone, prostate, and heart, and reaches high concentrations in bile. After a 4-g bolus injection, maximum biliary concentrations average 3,205 μg/mL. It penetrates into the cerebrospinal fluid in the presence of inflamed meninges.
Special Populations:
Renal Insufficiency: The elimination half-life is increased twofold in mild to moderate renal impairment and fivefold to sixfold in severe impairment. Because PIPRACIL is excreted by the biliary route as well as by the renal route, it can be used safely in appropriate dosage in patients with severely restricted kidney function. (See DOSAGE AND ADMINISTRATION.)
Pediatric Patients: After intravenous administration of 50 mg/kg (5-minute infusion) in neonates, the mean plasma concentration of piperacillin extrapolated to time zero was 141 μg/mL, and the apparent volume of distribution averaged 101 mL/kg.
In premature neonates, the mean elimination half-life has been reported to range from 147 to 258 minutes following administration of a single intravenous dose of 75 mg/kg, the half‑life decreasing with increasing postnatal age. The changes in half-life appeared to be caused by an immature renal system during the first weeks of life. In one study in neonates, the mean elimination half-life ranged from 127 to 217 minutes following a single intravenous dose of 50 mg/kg. As in premature neonates, the half-life in neonates decreased with increasing postnatal age. The mean total body clearance in neonates has been reported to range from 32 to 41 mL/min/1.73 m2 after an intravenous dose of 50 mg/kg.
Following administration of an intravenous dose of 50 mg/kg in older pediatric patients (from 1 month up to 15 years of age), the mean elimination half-life has been reported to range from 31 to 37 minutes, and the mean total body clearance has been reported to range from 124 to 160 mL/min/1.73 m2.
As in adults, PIPRACIL elimination tends to be prolonged in pediatric patients with renal impairment. In one study in pediatric patients (age range, 3.3 to 14.3 years), the mean elimination half-life in patients with decreased renal function was approximately 60 minutes versus 37 minutes in patients with normal renal function. The elimination half-life has been reported to range from 3.5 to 14 hours in neonates with severe renal impairment.
Pharmacokinetic data have indicated that among pediatric patients below 12 years of age, those with cystic fibrosis have increased bioavailability, lower serum concentrations, and increased total body clearance of piperacillin compared to young, healthy pediatric volunteers under 12 years of age.
-
MICROBIOLOGY
Piperacillin is an antibiotic which exerts its bactericidal activity by inhibiting both septum and cell wall synthesis. It is active against a variety of gram-positive and gram-negative aerobic and anaerobic bacteria. Piperacillin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic gram-positive microorganisms
Enterococci, including Enterococcus faecalis
Streptococcus pneumoniae
Streptococcus pyogenesAerobic gram-negative microorganisms
Acinetobacter species
Enterobacter species
Escherichia coli
Haemophilus influenzae (non-β-lactamase-producing strains)
Klebsiella species
Morganella morganii
Neisseria gonorrhoeae
Proteus mirabilis
Proteus vulgaris
Providencia rettgeri
Pseudomonas aeruginosa
Serratia speciesAnaerobic gram-positive microorganisms
Anaerobic cocci
Clostridium speciesAnaerobic gram-negative microorganisms
Bacteroides species, including Bacteroides fragilisThe following in vitro data are available, but their clinical significance is unknown.
At least 90% of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for piperacillin. However, the safety and effectiveness of piperacillin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Aerobic gram-positive microorganisms
Streptococcus agalactiae
Streptococcus bovis
Viridans group streptococciAerobic gram-negative microorganisms
Burkholderia cepacia
Citrobacter diversus
Citrobacter freundii
Pseudomonas fluorescens
Stenotrophomonas maltophilia
Yersinia enterocoliticaAnaerobic gram-positive microorganisms
Actinomyces species
Eubacterium speciesAnaerobic gram-negative microorganisms
Fusobacterium necrophorum
Fusobacterium nucleatum
Porphyromonas asaccharolytica
Prevotella melaninogenica
Veillonella speciesSusceptibility Testing Methods
Dilution Techniques:
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1,2 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of piperacillin powder. The MIC values should be interpreted according to the following criteria:
For testingEnterobacteriaceae and Acinetobacter species:
MIC (μg/mL) Interpretation ≤ 16
32-64
≥ 128Susceptible (S)
Intermediate (I)
Resistant (R)For testing Pseudomonas aeruginosa:
MIC (μg/mL) Interpretation ≤ 64
≥ 128Susceptible (S)
Resistant (R)For testing Enterococcus faecalisa:
MIC (μg/mL) Interpretation a Penicillin susceptibility may be used to predict the susceptibility to piperacillin.1,2
≤ 8
≥ 16Susceptible (S)
Resistant (R)Haemophilus species are considered susceptible if the MIC of piperacillin is≤ to 1 μg/mL.*
* Dilution methods such as those described in the International Collaborative Study (Acta Pathol Microbiol Scand [B] 1971; suppl 217) have been used to determine susceptibility of organisms to piperacillin.
Dilution (MICs) susceptibility test methods and interpretative criteria for assessing the susceptibility of Neisseria gonorrhoeae to piperacillin have not been established. However,β‑lactamase testing will detect one form of penicillin resistance in Neisseria gonorrhoeae and is recommended.1,2
Dilution (MICs) susceptibility test methods and interpretative criteria for assessing the susceptibility of Streptococcus pneumoniae and Streptococcus pyogenes to piperacillin have not been established.1,2
A report of“Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Quality Control:
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard piperacillin powder should provide the following MIC values:
Microorganism MIC (μg/mL) Enterococcus faecalis
Escherichia coli
Pseudomonas aeruginosaATCC 29212
ATCC 25922
ATCC 278531-4
1-4
1-8Diffusion Techniques:
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2,3 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 100μg of piperacillin to test the susceptibility of microorganisms to piperacillin.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 100 μg piperacillin disk should be interpreted according to the following criteria:
For testing Enterobacteriaceae and Acinetobacter species:
Zone Diameter (mm) Interpretation ≥ 21
18-20
≤ 17Susceptible (S)
Intermediate (I)
Resistant (R)For testing Pseudomonas aeruginosa:
Zone Diameter (mm) Interpretation ≥ 18
≤ 17Susceptible (S)
Resistant (R)For testing Enterococcus faecalisb:
Zone Diameter (mm) Interpretation b Penicillin susceptibility may be used to predict the susceptibility to piperacillin.2,3
≥ 15
≤ 14Susceptible (S)
Resistant (R)Haemophilus species which give zones of ≥ 29 mm are susceptible; resistant strains give zones of ≤ 28 mm. The above interpretive criteria are based on the use of the standardized procedure. Antibiotic susceptibility testing requires carefully prescribed procedures. Susceptibility tests are biased to a considerable degree when different methods are used.
NCCLS Approved Standard; M2-A2 (Formerly ASM-2) Performance Standards for Antimicrobic Disk Susceptibility Tests, Second Edition, available from the National Committee of Clinical Laboratory Standards.
Disk diffusion (zone diameters) susceptibility test methods and interpretative criteria for assessing the susceptibility of Neisseria gonorrhoeae to piperacillin have not been established. However, β-lactamase testing to penicillin is recommended. It will detect one form of penicillin resistance, chromosomally mediated resistance, in Neisseria gonorrhoeae. In addition, gonococci with 10-unit penicillin disk zone diameters of ≤ 19 mm are likely to be β-lactamase producing strains (plasmid-mediated penicillin resistance).2,3
Disk diffusion (zone diameters) susceptibility test methods and interpretative criteria for assessing the susceptibility of Streptococcus pneumoniae and Streptococcus pyogenes to piperacillin have not been established.2,3
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for piperacillin.
Quality Control:
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 100-μg piperacillin disk should provide the following zone diameters in these laboratory test quality control strains:Microorganism Zone Diameter (mm) Escherichia coli
Pseudomonas aeruginosaATCC 25922
ATCC 2785324-30
25-33Anaerobic Techniques:
For anaerobic bacteria, the susceptibility to piperacillin as MICs can be determined by standardized test methods.4 The MIC values obtained should be interpreted according to the following criteria:
MIC (μg/mL) Interpretation ≤ 32
64
≥ 128Susceptible (S)
Intermediate (I)
Resistant (R)Interpretation is identical to that stated above for results using dilution techniques.
As with other susceptibility techniques, the use of laboratory control microorganisms is required to control the technical aspects of the laboratory standardized procedures. Standardized piperacillin powder should provide the following MIC values:
Microorganism MIC (μg/mL) c This quality control range is applicable only to tests performed using either Brucella blood agar or Wilkins-Chalgren agar with the Reference Agar Dilution Method.4
d This quality range is applicable only to tests performed in the broth formulation of Wilkins-Chalgren agar with the Broth microdilution method.4Bacteroides fragilisc
Bacteroides thetaiotaomicrondATCC 25285
ATCC 297412-8
8-32 -
INDICATIONS AND USAGE
Therapeutic: PIPRACIL is indicated for the treatment of serious infections caused by susceptible strains of the designated microorganisms in the conditions listed below:
Intra-Abdominal Infections including hepatobiliary and surgical infections caused by E. coli, Pseudomonas aeruginosa, enterococci, Clostridium spp., anaerobic cocci, or Bacteroides spp., including B. fragilis.
Urinary Tract Infections caused by E. coli, Klebsiella spp., P. aeruginosa, Proteus spp., including P. mirabilis, or enterococci.
Gynecologic Infections including endometritis, pelvic inflammatory disease, pelvic cellulitis caused by Bacteroides spp., including B. fragilis, anaerobic cocci, Neisseria gonorrhoeae, or enterococci (E. faecalis).
Septicemia including bacteremia caused by E. coli, Klebsiella spp., Enterobacter spp., Serratia spp., P. mirabilis, S. pneumoniae, enterococci, P. aeruginosa, Bacteroides spp., or anaerobic cocci.
Lower RespiratoryTract Infections caused by E. coli, Klebsiella spp., Enterobacter spp., P. aeruginosa, Serratia spp., H. influenzae, Bacteroides spp., or anaerobic cocci. Although improvement has been noted in patients with cystic fibrosis, lasting bacterial eradication may not necessarily be achieved.
Skin and Skin Structure Infections caused by E. coli, Klebsiella spp., Serratia spp., Acinetobacter spp., Enterobacter spp., P. aeruginosa, Morganella morganii, Providencia rettgeri, Proteus vulgaris, P. mirabilis, Bacteroides spp., including B. fragilis, anaerobic cocci, or enterococci.
Bone and Joint Infections caused by P. aeruginosa, enterococci, Bacteroides spp., or anaerobic cocci.
Uncomplicated Gonococcal Urethritis caused by N. gonorrhoeae.
PIPRACIL has also been shown to be clinically effective for the treatment of infections at various sites caused by Streptococcus species including S. pyogenes and S. pneumoniae; however, infections caused by these organisms are ordinarily treated with more narrow spectrum penicillins. Because of its broad spectrum of bactericidal activity against gram-positive and gram-negative aerobic and anaerobic bacteria, PIPRACIL is particularly useful for the treatment of mixed infections and presumptive therapy prior to the identification of the causative organisms.
Also, PIPRACIL may be administered as single drug therapy in some situations where normally two antibiotics might be employed.
Piperacillin has been successfully used with aminoglycosides, especially in patients with impaired host defenses. Both drugs should be used in full therapeutic doses.
Appropriate cultures should be made for susceptibility testing before initiating therapy and therapy adjusted, if appropriate, once the results are known.
Prophylaxis: PIPRACIL is indicated for prophylactic use in surgery including intra-abdominal (gastrointestinal and biliary) procedures, vaginal hysterectomy, abdominal hysterectomy, and cesarean section. Effective prophylactic use depends on the time of administration; PIPRACIL should be given one-half to one hour before the operation so that effective levels can be achieved in the site prior to the procedure.
The prophylactic use of piperacillin should be stopped within 24 hours, since continuing administration of any antibiotic increases the possibility of adverse reactions, but in the majority of surgical procedures, does not reduce the incidence of subsequent infections. If there are signs of infection, specimens for culture and susceptibility testing should be obtained for identification of the causative microorganism so that appropriate therapy can be instituted.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of PIPRACIL and other antibacterial drugs, PIPRACILshould only be used to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC/ANAPHYLACTOID) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY AND/OR A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE INITIATING THERAPY WITH PIPRACIL, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, PIPRACIL SHOULD BE DISCONTINUED AND APPROPRIATE THERAPY INSTITUTED. SERIOUS ANAPHYLACTIC/ANAPHYLACTOID REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including PIPRACIL, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
Bleeding manifestations have occurred in some patients receiving β-lactam antibiotics, including piperacillin. These reactions have sometimes been associated with abnormalities of coagulation tests such as clotting time, platelet aggregation, and prothrombin time and are more likely to occur in patients with renal failure.
If bleeding manifestations occur, the antibiotic should be discontinued and appropriate therapy instituted.
The possibility of the emergence of resistant organisms which might cause superinfections should be kept in mind, particularly during prolonged treatment. If this occurs, appropriate measures should be taken.
As with other penicillins, patients may experience neuromuscular excitability or convulsions if higher than recommended doses are given intravenously.
PIPRACIL is a monosodium salt containing 1.85 mEq of Na+ per g (42.5 mg of Na+ per g). This should be considered when treating patients requiring restricted salt intake. Periodic electrolyte determinations should be made in patients with low potassium reserves, and the possibility of hypokalemia should be kept in mind with patients who have potentially low potassium reserves and who are receiving cytotoxic therapy or diuretics.
Leukopenia and neutropenia may occur during prolonged therapy.
As with other semisynthetic penicillins, PIPRACIL therapy has been associated with an increased incidence of fever and rash in cystic fibrosis patients.
Prescribing PIPRACIL in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Patients should be counseled that antibacterial drugs including PIPRACIL should only be used to treat bacterial infections. They do not treat viral infections (eg, the common cold). When PIPRACIL is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by PIPRACIL or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of antibiotic. If this occurs, patients should contact their physician as soon as possible.
Laboratory Tests
While piperacillin possesses the characteristic low toxicity of the penicillin group of antibiotics, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, during prolonged therapy is advisable.
All patients with gonorrhea should have a serologic test for syphilis at the time of diagnosis. Patients treated with piperacillin should have a follow-up serologic test for syphilis after 3 months.
Drug Interactions
Aminoglycosides
The mixing of piperacillin with an aminoglycoside in vitro can result in substantial inactivation of the aminoglycoside.
Vecuronium
When used in the perioperative period, piperacillin has been implicated in the prolongation of the neuromuscular blockade of vecuronium. Caution is indicated when piperacillin is used perioperatively. In one controlled clinical study, the ureidopenicillins, including piperacillin, were reported to prolong the action of vecuronium. Due to their similar mechanism of action, it is expected that the neuromuscular blockade produced by any of the non-depolarizing muscle relaxants could be prolonged in the presence of piperacillin.
Probenecid
The oral combination of probenecid before intramuscular injection of PIPRACIL produces an increase in piperacillin peak serum level of about 30%.
Drug/Laboratory Test Interactions
As with other penicillins, the administration of PIPRACIL may result in a false-positive reaction for glucose in the urine using a copper-reduction method. It is recommended that glucose tests based on enzymatic glucose oxidase reactions be used.
There have been reports of positive test results using the Bio-Rad Laboratories Platelia Aspergillus EIA test in patients receiving piperacillin/tazobactam injection who were subsequently found to be free of Aspergillus infection. Cross-reactions with non-Aspergillus polysaccharides and polyfuranoses with the Bio-Rad Laboratories Platelia Aspergillus EIA test have been reported.
Therefore, positive test results in patients receiving piperacillin should be interpreted cautiously and confirmed by other diagnostic methods.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Mutagenesis
Piperacillin was negative in the Ames Salmonella reversion test at concentrations up to 10 μg/plate. There was no DNA damage in bacteria (Rec assay) exposed to piperacillin at concentrations up to 200 μg/disc. In a mammalian point mutation (mouse lymphoma cells) assay, piperacillin was positive at concentrations ≥ 2500 μg/mL. Piperacillin was negative in a cell (BALB/c-3T3) transformation assay at concentrations up to 3000 μg/mL. In vivo, piperacillin did not induce chromosomal aberrations in the bone marrow cells of mice at I.V. doses up to 2000 mg/kg/day or rats at I.V. doses up to 1500 mg/kg/day. These doses are half (mice) or three‑fourths (rats) the maximum recommended human daily dose based on body-surface area (mg/m2). In another in vivo test, there was no dominant lethal effect when piperacillin was administered to rats at I.V. doses up to 2000 mg/kg/day, which is similar to the maximum recommended human daily dose based on body-surface area (mg/m2). When piperacillin was administered to mice at I.V. doses up to 2000 mg/kg/day, which is half the maximum recommended human daily dose based on body-surface area (mg/m2), urine from these animals was not mutagenic when tested in the Ames assay using Salmonella strain TA-98 in the absence of β-glucuronidase.
Impairment of Fertility
Reproduction studies have been performed in mice (SQ) and rats (I.P.) and have revealed no evidence of impaired fertility due to piperacillin administered up to a dose which is half (mice) or similar (rats) to the maximum recommended human daily dose based on body-surface area (mg/m2). The plasma/serum concentrations at the highest daily dose administered to rats in reproduction studies were comparable to the maximum serum concentration seen in man, based on a toxicology study in rats in which similar doses of piperacillin (in combination with a beta‑lactamase inhibitor, tazobactam) were administered I.P.and based on extrapolations from an animal pharmacokinetic study using lower doses of piperacillin alone.
Pregnancy
Teratogenic effects—Pregnancy Category B
Teratology studies have been performed in mice (I.V.) and rats (I.V., I.P. and SQ) and have revealed no evidence of harm to the fetus due to piperacillin administered up to a dose which is approximately half the maximum recommended human daily dose based on body-surface area (mg/m2). In pharmacokinetic studies in pregnant and nonpregnant rats, in which piperacillin was administered I.V. at a dose which is half the maximum daily dose administered in teratology studies, serum concentrations in rats were approximately 10 times the maximum serum concentration seen in man. In other studies in mice and rats, in which piperacillin (in combination with a beta-lactamase inhibitor, tazobactam) was administered I.V. at approximately half the maximum daily dose administered in teratology studies, plasma concentrations of piperacillin were approximately 2 times (mice) and 5 times (rats) the serum concentrations seen in man.
There are, however, no adequate and well-controlled studies with piperacillin in pregnant women. Because animal reproduction studies are not always predictive of the human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Piperacillin is excreted in low concentrations in human milk. Caution should be exercised when PIPRACIL is administered to nursing mothers.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Data from published pharmacokinetics studies indicate that the elimination half-life of piperacillin in neonates is twofold to fourfold longer than that seen in pediatric patients 1 month of age and above as well as in adults. In infants, children, and adolescents, the elimination half-life of piperacillin is shorter than that observed in adults. As in adults, the elimination of piperacillin is decreased in pediatric patients with renal impairment. (See CLINICAL PHARMACOLOGY.)
Geriatric Use
Clinical studies of PIPRACIL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
PIPRACIL contains 42.5 mg (1.85 mEq) of sodium per gram. At the usual recommended doses, patients would receive between 255 and 765 mg/day (11.1 and 33.3 mEq) of sodium. The geriatric population may respond with a blunted natriuresis to salt loading. The total sodium content from dietary and non-dietary sources may be clinically important with regard to such diseases as congestive heart failure.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
PIPRACIL is generally well tolerated. The most common adverse reactions have been local in nature, following intravenous or intramuscular injection. The following adverse reactions may occur:
Local Reactions: In clinical trials thrombophlebitis was noted in 4% of patients. Pain, erythema, and/or induration at the injection site occurred in 2% of patients. Less frequent reactions including ecchymosis, deep vein thrombosis, and hematomas have also occurred.
Gastrointestinal: Diarrhea and loose stools were noted in 2% of patients. Other less frequent reactions included vomiting, nausea, increases in liver enzymes (LDH, AST, ALT), hyperbilirubinemia, cholestatic hepatitis, bloody diarrhea, and pseudomembranous colitis. The onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment. (See WARNINGS.)
Hypersensitivity Reactions: Anaphylactic/anaphylactoid reactions (some leading to shock and fatalities) have been reported. (See WARNINGS.)
Rash was noted in 1% of patients. Other less frequent findings included pruritus, vesicular eruptions, and positive Coombs tests.
Other dermatologic manifestations, such as erythema multiforme, urticaria, toxic epidermal necrolysis and Stevens-Johnson syndrome, have been reported.
Renal: Elevations of creatinine or BUN, renal failure and interstitial nephritis have been reported.
Central Nervous System: Headache, dizziness, fatigue, and seizures have been reported.
Hemic and Lymphatic: Hemolytic anemia, agranulocytosis, pancytopenia, prolonged bleeding time, reversible leukopenia, neutropenia, thrombocytopenia, and/or eosinophilia have been reported. As with other β-lactam antibiotics, reversible leukopenia (neutropenia) is more apt to occur in patients receiving prolonged therapy at high dosages or in association with drugs known to cause this reaction.
Serum Electrolytes: Individuals with liver disease or individuals receiving cytotoxic therapy or diuretics were reported to demonstrate a decrease in serum potassium concentrations with high doses of piperacillin. Hypokalemia has been reported.
Skeletal: Prolonged muscle relaxation (see PRECAUTIONS, Drug Interactions).
Other: Fever, superinfection, including candidiasis; hemorrhagic manifestations have been reported.
Piperacillin therapy has been associated with an increased incidence of fever and rash in cystic fibrosis patients.
-
OVERDOSAGE
There is no specific information on overdose with PIPRACIL. Other penicillin-class drugs in overdosage, however, have the potential to cause neuromuscular hyperirritability or convulsive seizures. In case of overdosage, discontinue medication, treat symptomatically, and institute supportive measures as required. Piperacillin can be removed by hemodialysis but not peritoneal dialysis.
-
DOSAGE AND ADMINISTRATION
PIPRACIL may be administered by the intramuscular route (see NOTE) or intravenously as a three- to five-minute intravenous injection or as a 20- to 30-minute infusion. The usual dosage of PIPRACIL for serious infections is 3 to 4 g given every four to six hours as a 20- to 30-minute infusion. For serious infections, the intravenous route should be used.
PIPRACIL should not be mixed with an aminoglycoside in a syringe or infusion bottle since this can result in inactivation of the aminoglycoside.
The maximum daily dose for adults is usually 24 g/day, although higher doses have been used.
Intramuscular injections (see NOTE) should be limited to 2 g per injection site. This route of administration has been used primarily in the treatment of patients with uncomplicated gonorrhea and urinary tract infections.
DOSAGE RECOMMENDATIONS Type of Infection Usual Total Daily Dose †One g of probenecid should be given orally one-half hour prior to injection.
Serious infections such as septicemia, nosocomial pneumonia, intra-abdominal infections, aerobic and anaerobic gynecologic infections, and skin and soft tissue infections
12 – 18 g/d I.V. (200 – 300 mg/kg/d) in divided doses every 4 to 6 h Complicated urinary tract infections 8 – 16 g/d I.V. (125 – 200 mg/kg/d) in divided doses every 6 to 8 h Uncomplicated urinary tract infections and most community-acquired pneumonia 6 – 8 g/d I.M. or I.V. (100– 125 mg/kg/d) in divided doses every 6 to 12 h Uncomplicated gonorrhea infections 2 g I.M.† as a one-time dose The average duration of PIPRACIL treatment is from seven to ten days, except in the treatment of gynecologic infections, which is from three to ten days; the duration should be guided by the patient's clinical and bacteriological progress. For most acute infections, treatment should be continued for at least 48 to 72 hours after the patient becomes asymptomatic. Antibiotic therapy for S. pyogenes infections should be maintained for at least ten days to reduce the risk of rheumatic fever.
When PIPRACIL is given concurrently with aminoglycosides, both drugs should be used in full therapeutic doses.
Renal Impairment
Dosage in Renal Impairment Creatinine
Clearance
mL/minUrinary Tract
Infection
(uncomplicated)Urinary Tract
Infection
(complicated)Serious
Systemic
Infection>40 No dosage adjustment necessary 20-40 No dosage adjustment necessary 9 g/day
3 g every 8 h12 g/day
4 g every 8 h<20 6 g/day
3 g every 12 h6 g/day
3 g every 12 h8 g/day
4 g every 12 hFor patients on hemodialysis, the maximum daily dose is 6 g/day (2 g every 8 hours). In addition, because hemodialysis removes 30% to 50% of piperacillin in 4 hours, a 1-g additional dose should be administered following each dialysis period.
For patients with renal failure and hepatic insufficiency, measurement of serum levels of piperacillin will provide additional guidance for adjusting dosage.
Prophylaxis
When possible, PIPRACIL should be administered as a 20- to 30-minute infusion just prior to anesthesia. Administration while the patient is awake will facilitate identification of possible adverse reactions during drug infusion. (See PRECAUTION, Drug Interactions.)
INDICATION 1st Dose 2nd Dose 3rd Dose Intra-abdominal
Surgery2 g I.V. just prior to surgery 2 g during surgery 2 g every 6 h Post-Op for no more than 24 h Vaginal
Hysterectomy2 g I.V. just prior to surgery 2 g 6 h after 1st dose 2 g 12 h after 1st dose Cesarean
Section2 g I.V. after cord is clamped 2 g 4 h after 1st dose 2 g 8 h after 1st dose Abdominal
Hysterectomy2 g I.V. just prior to surgery 2 g on return to recovery room 2 g after 6 h Pediatric patients. Dosages in pediatric patients under 12 years of age have not been studied in adequate and well-controlled clinical trials (See CLINICAL PHARMACOLOGY).
¶Either Parabens or Benzyl Alcohol.
#For Intramuscular Use Only. Lidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type.PRODUCT RECONSTITUTION/DOSAGE PREPARATION Conventional Vials: Diluents for Reconstitution Sterile Water for Injection
Bacteriostatic¶ Water for InjectionSodium Chloride Injection
Bacteriostatic¶ Sodium Chloride Injection
Dextrose 5% in Water
Dextrose 5% and 0.9% Sodium Chloride
Lidocaine# HCl 0.5-1% (without epinephrine)††When PIPRACIL®is further diluted with Lactated Ringer's Injection, the diluted solution must be administered within 2 hours.
Conventional Vials: Intravenous Solutions Intravenous Admixtures Dextrose 5% in Water
0.9% Sodium Chloride
Dextrose 5% and 0.9% Sodium Chloride
Lactated Ringer's Injection††
Dextran 6% in 0.9% Sodium ChlorideNormal Saline [+ KCl 40 mEq]
5% Dextrose in Water [+ KCl 40 mEq]
5% Dextrose/Normal Saline [+ KCl 40 mEq]
Ringer's Injection [+ KCl 40 mEq]
Lactated Ringer's Injection [+ KCl 40 mEq]††Intravenous Administration
Reconstitution Directions for Conventional Vials: Reconstitute each gram of PIPRACIL with at least 5 mL of a suitable diluent (except Lidocaine HCl 0.5%-1% without epinephrine) listed above. Shake well until dissolved. Reconstituted solution may be diluted to the desired volume (eg, 50 or 100 mL) in the above listed intravenous solutions and admixtures.
DIRECTIONS FOR ADMINISTRATION
Intermittent IV Infusion
Infuse diluted solution over period of about 30 minutes. During infusion, it is desirable to discontinue the primary intravenous solution.Intravenous Injection (Bolus)
Reconstituted solution should be injected slowly over a 3-to 5-minute period to help avoid vein irritation.Intramuscular Administration
(Conventional Vials Only)Reconstitution Directions:Reconstitute each gram of PIPRACIL with 2 mL of a suitable diluent listed above to achieve a concentration of 1 g per 2.5 mL. Shake well until dissolved.
DIRECTIONS FOR ADMINISTRATION
When indicated by clinical and bacteriological findings, intramuscularadministration of 6 to 8 g daily of PIPRACIL, in divided doses, may be utilized for initiation of therapy. In addition, intramuscular administration of the drug may be considered for maintenance therapy after clinical and bacteriologic improvement has been obtained with intravenous piperacillin sodium treatment. Intramuscular administration should not exceed 2 g per injection at any one site.The preferred site is the upper outer quadrant of the buttock (ie, gluteus maximus).
The deltoid area should be used only if well-developed, and then only with caution to avoid radial nerve injury. Intramuscular injections should not be made into the lower or mid-third of the upper arm.
Stability of PIPRACIL Following Reconstitution
PIPRACIL is stable in both glass and plastic containers when reconstituted with recommended diluents and when diluted with the intravenous solutions and intravenous admixtures indicated above.
Pharmacy vials should be used immediately after reconstitution. Discard any unused portion after 24 hours if stored at room temperature (20° to 25°C [68° to 77°F]), or after 48 hours if stored at refrigerated temperature (2° to 8°C [36° to 46°F]). Vials should not be frozen after reconstitution.
-
How Supplied
PIPRACIL® (piperacillin for injection) is available in vials containing freeze-dried piperacillin sodium powder equivalent to two, three, and four g of piperacillin. One g of piperacillin (as a monosodium salt) contains 1.85 mEq (42.5 mg) of sodium.
Product Numbers
2 gram/Vial-10 per box-NDC: 0206-3879-16
3 gram/Vial-10 per box-NDC: 0206-3882-55
4 gram/Vial-10 per box-NDC 0206-3880-25Store at controlled room temperature 20°C-25°C(68°F-77°F).
-
REFERENCES
1National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically – Fifth Edition. Approved Standard NCCLS Document M7-A5, Vol. 20, No. 2, NCCLS, Wayne, PA, January, 2000.
2National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing – Eleventh Informational Supplement. NCCLS Document M100-S11, Vol. 21, No. 1, NCCLS, Wayne, PA, January, 2001.
3National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests – Seventh Edition. Approved Standard NCCLS Document M2-A7, Vol. 20, No. 1, NCCLS, Wayne, PA, January, 2000.
4National Committee for Clinical Laboratory Standards. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria – Fifth Edition. Approved Standard NCCLS Document M11-A5, Vol. 21, No. 2, NCCLS, Wayne, PA, March, 2001.
Wyeth®
Wyeth Pharmaceuticals Inc.
Philadelphia, PA 19101W10440C007
ET01
Rev 03/07 -
INGREDIENTS AND APPEARANCE
PIPRACIL
piperacillin sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0206-3879 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Piperacillin Sodium (UNII: M98T69Q7HP) (Piperacillin - UNII:X00B0D5O0E) 2 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0206-3879-16 10 in 1 CARTON 1 1 in 1 VIAL PIPRACIL
piperacillin sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0206-3882 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Piperacillin Sodium (UNII: M98T69Q7HP) (Piperacillin - UNII:X00B0D5O0E) 3 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0206-3882-55 10 in 1 CARTON 1 1 in 1 VIAL PIPRACIL
piperacillin sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0206-3880 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Piperacillin Sodium (UNII: M98T69Q7HP) (Piperacillin - UNII:X00B0D5O0E) 4 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0206-3880-25 10 in 1 CARTON 1 1 in 1 VIAL Labeler - Wyeth Pharmaceuticals, Inc.
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.