ValueStim
GUDID 00850958007473
2"x 2" Cloth 2 pk
Compass Richmar, LLC
Transcutaneous electrical stimulation electrode| Primary Device ID | 00850958007473 |
| NIH Device Record Key | 585b7b14-23d6-465b-89b9-44897c9ae26c |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ValueStim |
| Version Model Number | 203-835 |
| Company DUNS | 080930531 |
| Company Name | Compass Richmar, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850958007473 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K050469
FDA Product Code
| GXY | Electrode, Cutaneous |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2016-09-01 |
On-Brand Devices [ValueStim]
| 80092237901034 | 2"x 3.5" Cloth |
| 80092237901027 | 2"x 2" Cloth |
| 00850958007473 | 2"x 2" Cloth 2 pk |
| 00850689007452 | 2"x 3.5" Cloth Silver |
| 00850689007445 | 2"x 3.5" Cloth |
| 00850689007438 | 2"x 2" Cloth Silver |
| 00850689007421 | 2"x 2" Cloth |
| 10850958007470 | 2"x 2" Cloth 2 pk, Case of 10 |
| 10850689007442 | 2"x 3.5" Cloth, Case of 10 |
| 10850689007428 | 2"x 2" Cloth, Case of 10 |
Trademark Results [ValueStim]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
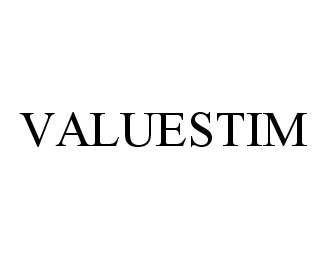 VALUESTIM 78462030 not registered Dead/Abandoned |
ENCORE MEDICAL ASSET CORPORATION 2004-08-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.