NDC 0009-0068
Xanax XR
Alprazolam
Xanax XR is a Oral Tablet, Extended Release in the Human Prescription Drug category. It is labeled and distributed by Pharmacia & Upjohn Company Llc. The primary component is Alprazolam.
| Product ID | 0009-0068_3889a3af-7247-42d4-ae83-aaee653ae3ba |
| NDC | 0009-0068 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Xanax XR |
| Generic Name | Alprazolam |
| Dosage Form | Tablet, Extended Release |
| Route of Administration | ORAL |
| Marketing Start Date | 2003-01-17 |
| Marketing Category | NDA / NDA |
| Application Number | NDA021434 |
| Labeler Name | Pharmacia & Upjohn Company LLC |
| Substance Name | ALPRAZOLAM |
| Active Ingredient Strength | 3 mg/1 |
| Pharm Classes | Benzodiazepine [EPC],Benzodiazepines [CS] |
| DEA Schedule | CIV |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 0009-0068-07
60 TABLET, EXTENDED RELEASE in 1 BOTTLE (0009-0068-07)
| Marketing Start Date | 2003-01-17 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 0009-0068-07 [00009006807]
Xanax XR TABLET, EXTENDED RELEASE
| Marketing Category | NDA |
| Application Number | NDA021434 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2003-01-17 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| ALPRAZOLAM | 3 mg/1 |
OpenFDA Data
| SPL SET ID: | aa58fff3-c297-49be-838b-599c32ad9835 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| UPC Code |
Pharmacological Class
- Benzodiazepine [EPC]
- Benzodiazepines [CS]
NDC Crossover Matching brand name "Xanax XR" or generic name "Alprazolam"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0054-3068 | Alprazolam | Alprazolam |
| 0228-2027 | Alprazolam | Alprazolam |
| 0009-0029 | Xanax | alprazolam |
| 0009-0055 | Xanax | alprazolam |
| 0009-0057 | Xanax | alprazolam |
| 0009-0059 | Xanax | alprazolam |
| 0009-0066 | Xanax | alprazolam |
| 0009-0068 | Xanax | alprazolam |
| 0009-0090 | Xanax | alprazolam |
| 0009-0094 | Xanax | alprazolam |
Trademark Results [Xanax]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
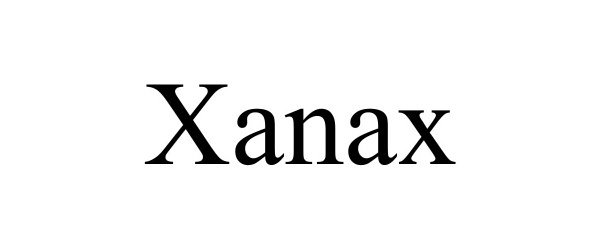 XANAX 98640047 not registered Live/Pending |
Ghost Global LLC 2024-07-09 |
 XANAX 74527349 1911413 Live/Registered |
Kabushiki Kaisha Xanax 1994-05-23 |
 XANAX 74106690 1764575 Live/Registered |
Kabushiki Kaisha Xanax 1990-10-17 |
 XANAX 73832739 1596581 Dead/Cancelled |
KABUSHIKI KAISHA XANAX (XANAX CORPORATION) 1989-10-20 |
 XANAX 73832696 not registered Dead/Abandoned |
KABUSHIKI KAISHA XANAX (XANAX CORPORATION) 1989-10-20 |
 XANAX 73832693 1630811 Dead/Cancelled |
KABUSHIKI KAISHA XANAX (XANAX CORPORATION) 1989-10-20 |
 XANAX 73560909 not registered Dead/Abandoned |
NANIWA CORPORATION (NANIWA KABUSHIKI KAISHA) 1985-09-30 |
 XANAX 73172914 1137561 Live/Registered |
UPJOHN COMPANY, THE 1978-06-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.