NDC 0078-0563
Exforge HCT
Amlodipine Valsartan And Hydrochlorothiazide
Exforge HCT is a Oral Tablet, Film Coated in the Human Prescription Drug category. It is labeled and distributed by Novartis Pharmaceuticals Corporation. The primary component is Amlodipine Besylate; Valsartan; Hydrochlorothiazide.
| Product ID | 0078-0563_10b0a131-e828-48e1-9e2b-92a6bbcb753a |
| NDC | 0078-0563 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Exforge HCT |
| Generic Name | Amlodipine Valsartan And Hydrochlorothiazide |
| Dosage Form | Tablet, Film Coated |
| Route of Administration | ORAL |
| Marketing Start Date | 2009-04-04 |
| Marketing Category | NDA / NDA |
| Application Number | NDA022314 |
| Labeler Name | Novartis Pharmaceuticals Corporation |
| Substance Name | AMLODIPINE BESYLATE; VALSARTAN; HYDROCHLOROTHIAZIDE |
| Active Ingredient Strength | 10 mg/1; mg/1; mg/1 |
| Pharm Classes | Calcium Channel Antagonists [MoA],Dihydropyridine Calcium Channel Blocker [EPC],Dihydropyridines [CS],Angiotensin 2 Receptor Antagonists [MoA],Angiotensin 2 Receptor Blocker [EPC],Increased Diuresis [PE],Thiazide Diuretic [EPC],Thiazides [CS] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 0078-0563-15
30 TABLET, FILM COATED in 1 BOTTLE (0078-0563-15)
| Marketing Start Date | 2009-04-04 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 0078-0563-34 [00078056334]
Exforge HCT TABLET, FILM COATED
| Marketing Category | NDA |
| Application Number | NDA022314 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 2009-04-04 |
| Marketing End Date | 2014-06-19 |
NDC 0078-0563-30 [00078056330]
Exforge HCT TABLET, FILM COATED
| Marketing Category | NDA |
| Application Number | NDA022314 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2009-04-04 |
| Marketing End Date | 2019-04-30 |
NDC 0078-0563-15 [00078056315]
Exforge HCT TABLET, FILM COATED
| Marketing Category | NDA |
| Application Number | NDA022314 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2009-04-04 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| AMLODIPINE BESYLATE | 10 mg/1 |
OpenFDA Data
| SPL SET ID: | 18d7820d-471f-4ee2-9ec6-25d8d27c77de |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| UPC Code |
Pharmacological Class
- Calcium Channel Antagonists [MoA]
- Dihydropyridine Calcium Channel Blocker [EPC]
- Dihydropyridines [CS]
- Angiotensin 2 Receptor Antagonists [MoA]
- Angiotensin 2 Receptor Blocker [EPC]
- Increased Diuresis [PE]
- Thiazide Diuretic [EPC]
- Thiazides [CS]
NDC Crossover Matching brand name "Exforge HCT" or generic name "Amlodipine Valsartan And Hydrochlorothiazide"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0078-0559 | Exforge HCT | amlodipine valsartan and hydrochlorothiazide |
| 0078-0560 | Exforge HCT | amlodipine valsartan and hydrochlorothiazide |
| 0078-0561 | Exforge HCT | amlodipine valsartan and hydrochlorothiazide |
| 0078-0562 | Exforge HCT | amlodipine valsartan and hydrochlorothiazide |
| 0078-0563 | Exforge HCT | amlodipine valsartan and hydrochlorothiazide |
| 0781-5756 | AMLODIPINE, VALSARTAN, HYDROCHLOROTHIAZIDE | amlodipine valsartan and hydrochlorothiazide |
| 0781-5760 | AMLODIPINE, VALSARTAN, HYDROCHLOROTHIAZIDE | amlodipine valsartan and hydrochlorothiazide |
| 0781-5771 | AMLODIPINE, VALSARTAN, HYDROCHLOROTHIAZIDE | amlodipine valsartan and hydrochlorothiazide |
| 0781-5787 | AMLODIPINE, VALSARTAN, HYDROCHLOROTHIAZIDE | amlodipine valsartan and hydrochlorothiazide |
| 0781-5794 | AMLODIPINE, VALSARTAN, HYDROCHLOROTHIAZIDE | amlodipine valsartan and hydrochlorothiazide |
Trademark Results [Exforge HCT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
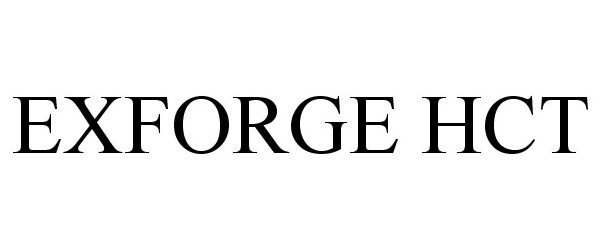 EXFORGE HCT 77202989 3923438 Live/Registered |
Novartis AG 2007-06-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.