NDC 0485-0151
UROGESIC BLUE
Methenamine, Sodium Phosphate, Monobasic, Methylene Blue, And Hyoscyamine Sulfate
UROGESIC BLUE is a Oral Tablet, Coated in the Human Prescription Drug category. It is labeled and distributed by Edwards Pharmaceuticals, Inc.. The primary component is Methenamine; Sodium Phosphate, Monobasic; Methylene Blue; Hyoscyamine Sulfate.
| Product ID | 0485-0151_10109075-6a32-4562-9dc8-c148a61d5d58 |
| NDC | 0485-0151 |
| Product Type | Human Prescription Drug |
| Proprietary Name | UROGESIC BLUE |
| Generic Name | Methenamine, Sodium Phosphate, Monobasic, Methylene Blue, And Hyoscyamine Sulfate |
| Dosage Form | Tablet, Coated |
| Route of Administration | ORAL |
| Marketing Start Date | 2010-11-19 |
| Marketing Category | UNAPPROVED DRUG OTHER / UNAPPROVED DRUG OTHER |
| Labeler Name | EDWARDS PHARMACEUTICALS, INC. |
| Substance Name | METHENAMINE; SODIUM PHOSPHATE, MONOBASIC; METHYLENE BLUE; HYOSCYAMINE SULFATE |
| Active Ingredient Strength | 82 mg/1; mg/1; mg/1; mg/1 |
| Pharm Classes | Osmotic Laxative [EPC],Increased Large Intestinal Motility [PE],Inhibition Large Intestine Fluid/Electrolyte Absorption [PE],Osmotic Activity [MoA],Oxidation-Reduction Activity [MoA],Oxidation-Reduction Agent [EPC] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 0485-0151-30
30 TABLET, COATED in 1 BOTTLE, PLASTIC (0485-0151-30)
| Marketing Start Date | 2010-11-19 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 0485-0151-30 [00485015130]
UROGESIC BLUE TABLET, COATED
| Marketing Category | unapproved drug other |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2010-11-19 |
NDC 0485-0151-01 [00485015101]
UROGESIC BLUE TABLET, COATED
| Marketing Category | unapproved drug other |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2010-11-19 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| METHENAMINE | 81.6 mg/1 |
OpenFDA Data
| SPL SET ID: | f650dd78-615c-42d3-9b2d-3a989039d80c |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Pharmacological Class
- Osmotic Laxative [EPC]
- Increased Large Intestinal Motility [PE]
- Inhibition Large Intestine Fluid/Electrolyte Absorption [PE]
- Osmotic Activity [MoA]
- Oxidation-Reduction Activity [MoA]
- Oxidation-Reduction Agent [EPC]
Trademark Results [UROGESIC BLUE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
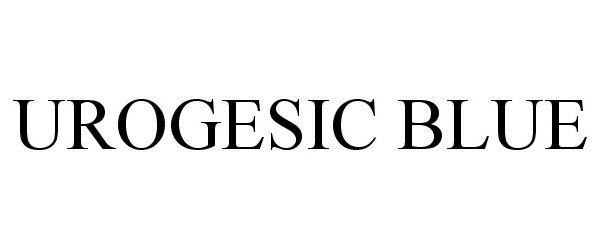 UROGESIC BLUE 86272498 4698652 Live/Registered |
Edwards Pharmaceuticals, Inc. 2014-05-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.