NDC 17312-002
Blur Relief
Jacobaea Maritima, Calcium Fluoride, Conium Maculatum Flowering Top, Euphrasia Stricta, Gelsemium Sempervirens Root, Sodium Chloride, Ruta Graveolens Flowering Top
Blur Relief is a Intraocular Liquid in the Human Otc Drug category. It is labeled and distributed by Trp Company. The primary component is Jacobaea Maritima; Calcium Fluoride; Conium Maculatum Flowering Top; Euphrasia Stricta; Gelsemium Sempervirens Root; Sodium Chloride; Ruta Graveolens Flowering Top.
| Product ID | 17312-002_4c218ce3-d82c-40df-8a60-3d536eb30879 |
| NDC | 17312-002 |
| Product Type | Human Otc Drug |
| Proprietary Name | Blur Relief |
| Generic Name | Jacobaea Maritima, Calcium Fluoride, Conium Maculatum Flowering Top, Euphrasia Stricta, Gelsemium Sempervirens Root, Sodium Chloride, Ruta Graveolens Flowering Top |
| Dosage Form | Liquid |
| Route of Administration | INTRAOCULAR |
| Marketing Start Date | 2006-06-01 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / UNAPPROVED HOMEOPATHIC |
| Labeler Name | TRP Company |
| Substance Name | JACOBAEA MARITIMA; CALCIUM FLUORIDE; CONIUM MACULATUM FLOWERING TOP; EUPHRASIA STRICTA; GELSEMIUM SEMPERVIRENS ROOT; SODIUM CHLORIDE; RUTA GRAVEOLENS FLOWERING TOP |
| Active Ingredient Strength | 5 [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 17312-002-11
1 BOTTLE, DROPPER in 1 PACKAGE (17312-002-11) > 15 mL in 1 BOTTLE, DROPPER
| Marketing Start Date | 2006-06-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 17312-002-11 [17312000211]
Blur Relief LIQUID
| Marketing Category | unapproved homeopathic |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2006-06-01 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| JACOBAEA MARITIMA | 5 [hp_X]/mL |
OpenFDA Data
| SPL SET ID: | 6c029a53-a4d1-4af6-8ce5-915a2bea67b2 |
| Manufacturer | |
| UNII | |
| UPC Code |
Trademark Results [Blur Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
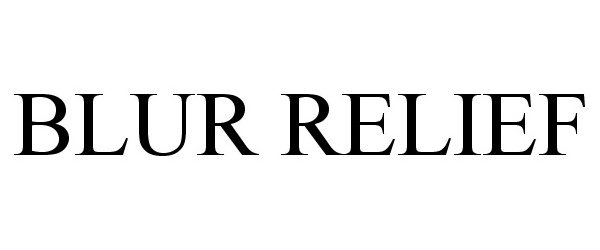 BLUR RELIEF 78789750 3170309 Live/Registered |
TRP Company 2006-01-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.