NDC 24571-117
PHOXILLUM B22K4/0
Magnesium Chloride, Sodium Chloride, Sodium Bicarbonate, Potassium Chloride And Sodium Phosphate Dibasic Dihydrate
PHOXILLUM B22K4/0 is a Intravenous Injection in the Human Prescription Drug category. It is labeled and distributed by Baxter Healthcare Corporation. The primary component is Magnesium Chloride; Potassium Chloride; Sodium Bicarbonate; Sodium Chloride; Sodium Phosphate, Dibasic, Dihydrate.
| Product ID | 24571-117_250f27ad-a700-4fd7-aaeb-7ca4211c17d8 |
| NDC | 24571-117 |
| Product Type | Human Prescription Drug |
| Proprietary Name | PHOXILLUM B22K4/0 |
| Generic Name | Magnesium Chloride, Sodium Chloride, Sodium Bicarbonate, Potassium Chloride And Sodium Phosphate Dibasic Dihydrate |
| Dosage Form | Injection |
| Route of Administration | INTRAVENOUS |
| Marketing Start Date | 2015-01-13 |
| Marketing Category | NDA / NDA |
| Application Number | NDA207026 |
| Labeler Name | Baxter Healthcare Corporation |
| Substance Name | MAGNESIUM CHLORIDE; POTASSIUM CHLORIDE; SODIUM BICARBONATE; SODIUM CHLORIDE; SODIUM PHOSPHATE, DIBASIC, DIHYDRATE |
| Active Ingredient Strength | 3 g/L; g/L; g/L; g/L; g/L |
| Pharm Classes | Calculi Dissolution Agent [EPC], Increased Large Intestinal Motility [PE], Increased Large Intestinal Motility [PE], Increased Large Intestinal Motility [PE], Increased Large Intestinal Motility [PE], Increased Large Intestinal Motility [PE], Inhibition Large Intestine Fluid/Electrolyte Absorption [PE], Inhibition Large Intestine Fluid/Electrolyte Absorption [PE], Inhibition Large Intestine Fluid/Electrolyte Absorption [PE], Inhibition Large Intestine Fluid/Electrolyte Absorption [PE], Inhibition Large Intestine Fluid/Electrolyte Absorption [PE], Inhibition Small Intestine Fluid/Electrolyte Absorption [PE], Magnesium Ion Exchange Activity [MoA], Osmotic Activity [MoA], Osmotic Activity [MoA], Osmotic Activity [MoA], Osmotic Activity [MoA], Osmotic Activity [MoA], Osmotic Laxative [EPC], Osmotic Laxative [EPC], Osmotic Laxative [EPC], Osmotic Laxative [EPC], Osmotic Laxative [EPC], Potassium Compounds [CS], Potassium Salt [EPC], Stimulation Large Intestine Fluid/Electrolyte Secretion [PE] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 24571-117-06
2 BAG in 1 CASE (24571-117-06) > 5 L in 1 BAG
| Marketing Start Date | 2015-01-13 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 24571-117-06 [24571011706]
PHOXILLUM B22K4/0 INJECTION
| Marketing Category | NDA |
| Application Number | NDA207026 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 2015-01-13 |
NDC 24571-117-05 [24571011705]
PHOXILLUM B22K4/0 INJECTION
| Marketing Category | NDA |
| Application Number | NDA207026 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 2015-01-13 |
| Marketing End Date | 2020-04-27 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| MAGNESIUM CHLORIDE | 3.05 g/L |
OpenFDA Data
| SPL SET ID: | 7f675fee-8940-4ece-bb0c-75ac79d8708c |
| Manufacturer | |
| UNII |
Pharmacological Class
- Calculi Dissolution Agent [EPC]
- Magnesium Ion Exchange Activity [MoA]
- Osmotic Laxative [EPC]
- Osmotic Activity [MoA]
- Inhibition Small Intestine Fluid/Electrolyte Absorption [PE]
- Increased Large Intestinal Motility [PE]
- Stimulation Large Intestine Fluid/Electrolyte Secretion [PE]
- Inhibition Large Intestine Fluid/Electrolyte Absorption [PE]
- Osmotic Laxative [EPC]
- Increased Large Intestinal Motility [PE]
- Inhibition Large Intestine Fluid/Electrolyte Absorption [PE]
- Osmotic Activity [MoA]
- Osmotic Laxative [EPC]
- Increased Large Intestinal Motility [PE]
- Inhibition Large Intestine Fluid/Electrolyte Absorption [PE]
- Osmotic Activity [MoA]
- Potassium Compounds [CS]
- Potassium Salt [EPC]
- Osmotic Laxative [EPC]
- Increased Large Intestinal Motility [PE]
- Inhibition Large Intestine Fluid/Electrolyte Absorption [PE]
- Osmotic Activity [MoA]
- Osmotic Laxative [EPC]
- Increased Large Intestinal Motility [PE]
- Inhibition Large Intestine Fluid/Electrolyte Absorption [PE]
- Osmotic Activity [MoA]
Trademark Results [PHOXILLUM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
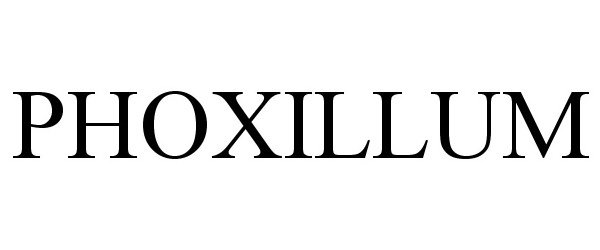 PHOXILLUM 86346945 4842319 Live/Registered |
GAMBRO LUNDIA AB 2014-07-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.