NDC 41250-506
Allergy Relief non drowsy
Fexofenadine Hcl
Allergy Relief non drowsy is a Oral Tablet, Coated in the Human Otc Drug category. It is labeled and distributed by Meijer, Inc.. The primary component is Fexofenadine Hydrochloride.
| Product ID | 41250-506_007d38da-2935-41bd-abaa-aaf87e1dc804 |
| NDC | 41250-506 |
| Product Type | Human Otc Drug |
| Proprietary Name | Allergy Relief non drowsy |
| Generic Name | Fexofenadine Hcl |
| Dosage Form | Tablet, Coated |
| Route of Administration | ORAL |
| Marketing Start Date | 2018-03-31 |
| Marketing Category | ANDA / ANDA |
| Application Number | ANDA204507 |
| Labeler Name | MEIJER, INC. |
| Substance Name | FEXOFENADINE HYDROCHLORIDE |
| Active Ingredient Strength | 180 mg/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 41250-506-15
15 BLISTER PACK in 1 CARTON (41250-506-15) > 1 TABLET, COATED in 1 BLISTER PACK
| Marketing Start Date | 2018-03-31 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 41250-506-15 [41250050615]
Allergy Relief non drowsy TABLET, COATED
| Marketing Category | ANDA |
| Application Number | ANDA204507 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2018-03-31 |
NDC 41250-506-90 [41250050690]
Allergy Relief non drowsy TABLET, COATED
| Marketing Category | ANDA |
| Application Number | ANDA204507 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2018-03-31 |
NDC 41250-506-30 [41250050630]
Allergy Relief non drowsy TABLET, COATED
| Marketing Category | ANDA |
| Application Number | ANDA204507 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2018-03-31 |
NDC 41250-506-45 [41250050645]
Allergy Relief non drowsy TABLET, COATED
| Marketing Category | ANDA |
| Application Number | ANDA204507 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2018-03-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| FEXOFENADINE HYDROCHLORIDE | 180 mg/1 |
OpenFDA Data
| SPL SET ID: | b3395454-65db-4d8b-abce-2475b049a114 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| UPC Code |
NDC Crossover Matching brand name "Allergy Relief non drowsy" or generic name "Fexofenadine Hcl"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 69230-312 | Allergy Relief Non drowsy | Allergy Relief Non drowsy |
| 41250-506 | Allergy Relief non drowsy | Allergy Relief non drowsy |
| 50090-4148 | Allergy Relief Non drowsy | Allergy Relief Non drowsy |
| 52904-430 | Allergy Relief Non Drowsy | Allergy Relief Non Drowsy |
| 69230-317 | Allergy Relief Non drowsy | Allergy Relief Non drowsy |
| 71335-1461 | Allergy Relief Non drowsy | Allergy Relief Non drowsy |
| 71335-1651 | Allergy Relief Non drowsy | Allergy Relief Non drowsy |
| 68788-7769 | Allergy Relief Non drowsy | Allergy Relief Non drowsy |
| 70934-685 | Allergy Relief Non drowsy | Allergy Relief Non drowsy |
| 50090-5067 | Allergy Relief Non drowsy | Allergy Relief Non drowsy |
| 10202-571 | 7 Select Allergy Relief | fexofenadine hcl |
| 0363-8010 | Allergy Relief | Fexofenadine HCl |
| 0904-6978 | Allergy Relief | Fexofenadine HCL |
| 11673-104 | Allergy Relief | Fexofenadine HCl |
| 0113-7425 | basic care allergy | Fexofenadine HCl |
| 0113-7571 | basic care allergy | fexofenadine hcl |
| 0363-9230 | Dye Free Wal Fex | Fexofenadine HCL |
| 0904-7192 | fexofenadine hcl | fexofenadine hcl |
| 0904-6979 | Fexofenadine Hydrochloride | Fexofenadine HCl |
| 0904-7050 | Fexofenadine Hydrochloride | Fexofenadine HCl |
| 10544-231 | Fexofenadine Hydrochloride | Fexofenadine HCl |
| 0113-0425 | Good Sense Aller Ease | Fexofenadine HCl |
| 0363-0904 | Indoor Outdoor Allergy Relief | Fexofenadine HCl |
| 0536-1066 | rugby fexofenadine hydrochloride | fexofenadine hcl |
| 0363-0903 | Wal Fex | Fexofenadine HCl |
| 0363-0600 | Wal Fex 24 Hour Allergy | fexofenadine hcl |
| 0363-0425 | Wal fex Allergy | Fexofenadine HCl |
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
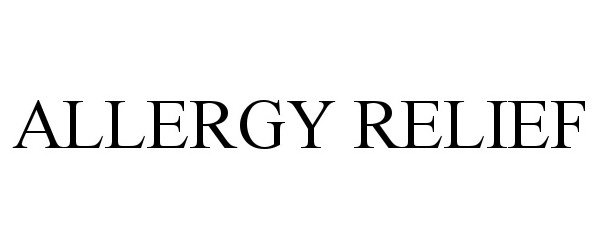 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
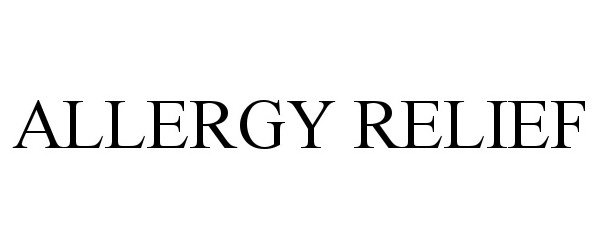 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
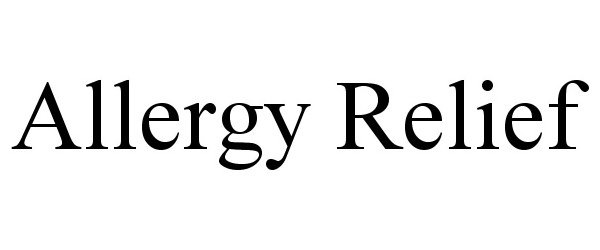 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
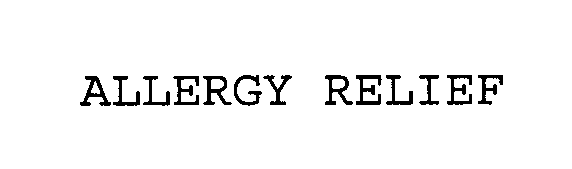 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.