NDC 51523-285
FACE IT 32800285
Octinoxate, Titanium Oxide And Zinc Oxide Cream
FACE IT 32800285 is a Topical Cream in the Human Otc Drug category. It is labeled and distributed by Thefaceshop North America, Inc.. The primary component is Octinoxate; Titanium Dioxide; Zinc Oxide.
| Product ID | 51523-285_07d4e9a6-8732-5c4f-e054-00144ff88e88 |
| NDC | 51523-285 |
| Product Type | Human Otc Drug |
| Proprietary Name | FACE IT 32800285 |
| Generic Name | Octinoxate, Titanium Oxide And Zinc Oxide Cream |
| Dosage Form | Cream |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2014-11-20 |
| Marketing Category | OTC MONOGRAPH NOT FINAL / OTC MONOGRAPH NOT FINAL |
| Application Number | part352 |
| Labeler Name | THEFACESHOP NORTH AMERICA, INC. |
| Substance Name | OCTINOXATE; TITANIUM DIOXIDE; ZINC OXIDE |
| Active Ingredient Strength | 2 g/40mL; g/40mL; g/40mL |
| NDC Exclude Flag | E |
| Listing Certified Through | 2017-12-31 |
Packaging
NDC 51523-285-03
1 TUBE in 1 CARTON (51523-285-03) > 40 mL in 1 TUBE
| Marketing Start Date | 2014-11-20 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 51523-285-03 [51523028503]
FACE IT 32800285 CREAM
| Marketing Category | OTC monograph not final |
| Application Number | part352 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2014-11-20 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| OCTINOXATE | 2.2 g/40mL |
OpenFDA Data
| SPL SET ID: | 07d4e9a6-8731-5c4f-e054-00144ff88e88 |
| Manufacturer | |
| UNII |
NDC Crossover Matching brand name "FACE IT 32800285" or generic name "Octinoxate, Titanium Oxide And Zinc Oxide Cream"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 51523-285 | FACE IT | octinoxate, titanium oxide and zinc oxide cream |
| 51523-379 | FACE IT BB (Natural Beige) | octinoxate, titanium oxide and zinc oxide cream |
Trademark Results [FACE IT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
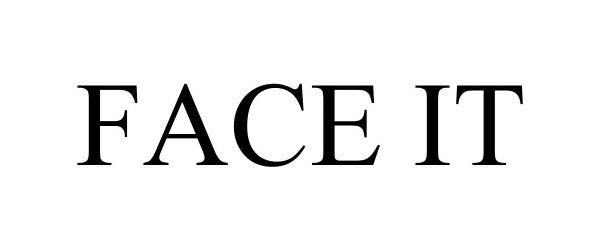 FACE IT 97697722 not registered Live/Pending |
Judy Thornton 2022-11-30 |
 FACE IT 88894535 not registered Live/Pending |
Suh, Joong Won 2020-04-30 |
 FACE IT 88215331 5763611 Live/Registered |
THEFACESHOP CO., LTD. 2018-12-03 |
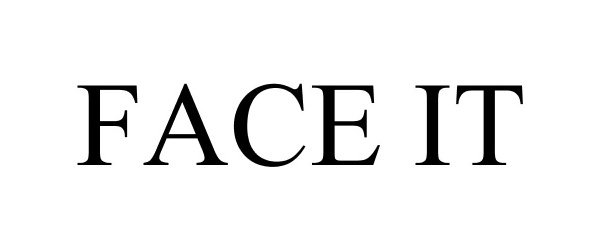 FACE IT 86973140 5423990 Live/Registered |
Garmin Switzerland GmbH 2016-04-12 |
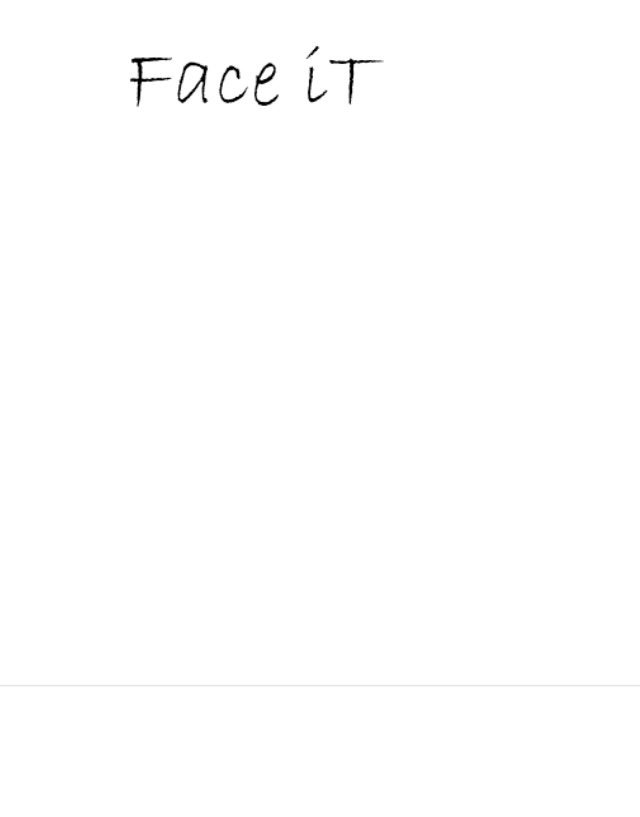 FACE IT 86531183 not registered Dead/Abandoned |
April McCoy-Henderson 2015-02-11 |
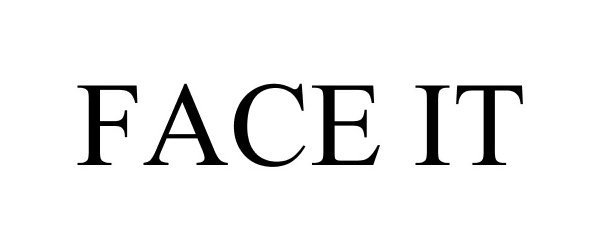 FACE IT 85920011 4451148 Live/Registered |
Kosair Charities Committee, Inc. 2013-05-01 |
 FACE IT 85896725 4720711 Live/Registered |
CELLULAR ETC. CORPORATION 2013-04-05 |
 FACE IT 85628369 not registered Dead/Abandoned |
Face It Advertising, Inc. 2012-05-17 |
 FACE IT 85285542 4432879 Live/Registered |
CLOTH IN A BOX INC. 2011-04-04 |
 FACE IT 85278091 4093303 Live/Registered |
Meier, Mark Alan 2011-03-27 |
 FACE IT 77796149 not registered Dead/Abandoned |
Reverta Health Solutions 2009-08-04 |
 FACE IT 76701852 3858661 Dead/Cancelled |
Alphabet Goods, LLC 2010-03-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.