NDC 53217-328
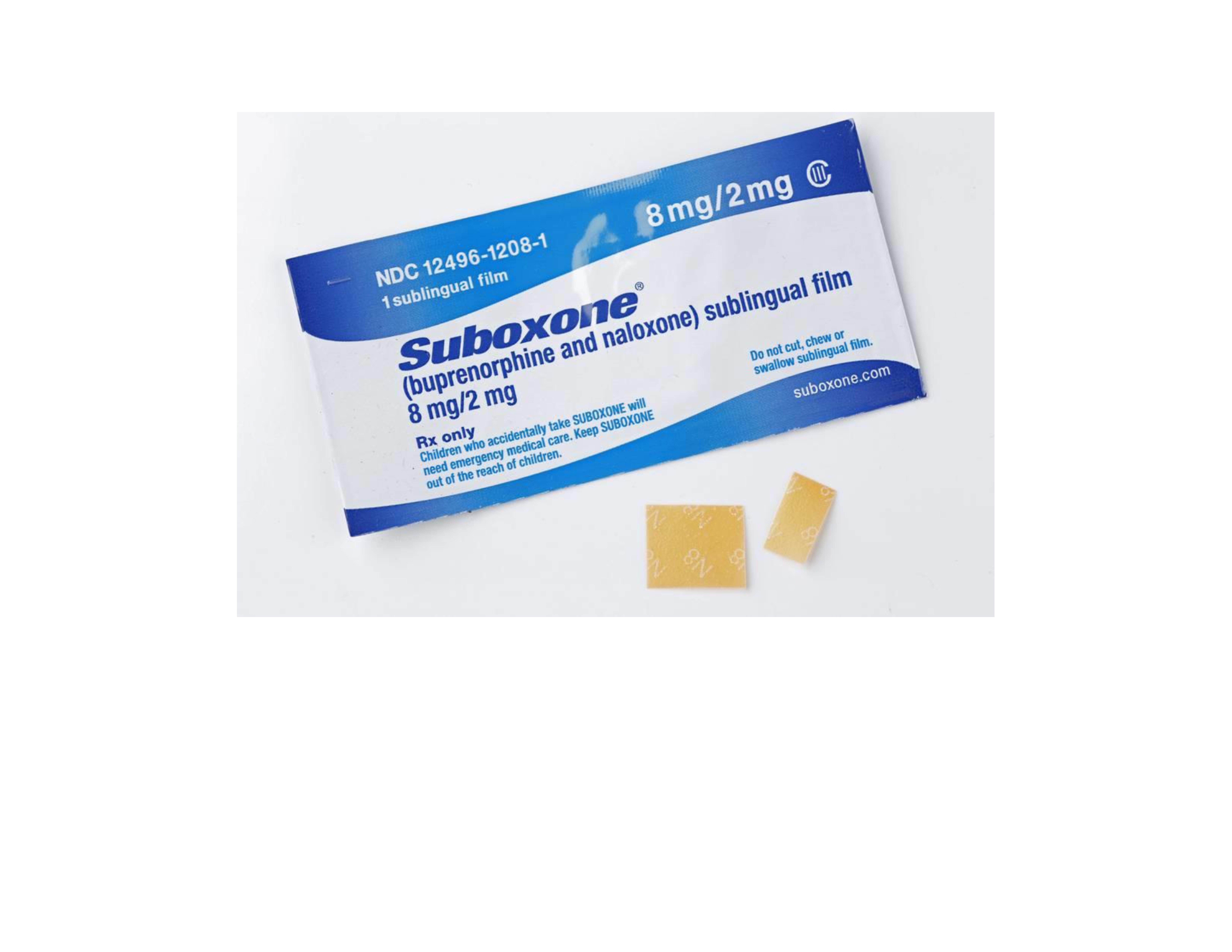
Suboxone
Buprenorphine Hydrochloride, Naloxone Hydrochloride
Suboxone is a Sublingual Film, Soluble in the Human Prescription Drug category. It is labeled and distributed by Aidarex Pharmaceuticals Llc. The primary component is Buprenorphine Hydrochloride; Naloxone Hydrochloride.
| Product ID | 53217-328_4f1210ae-a8ec-4253-b825-35e66ac93a1c |
| NDC | 53217-328 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Suboxone |
| Generic Name | Buprenorphine Hydrochloride, Naloxone Hydrochloride |
| Dosage Form | Film, Soluble |
| Route of Administration | SUBLINGUAL |
| Marketing Start Date | 2010-09-13 |
| Marketing Category | NDA / NDA |
| Application Number | NDA022410 |
| Labeler Name | Aidarex Pharmaceuticals LLC |
| Substance Name | BUPRENORPHINE HYDROCHLORIDE; NALOXONE HYDROCHLORIDE |
| Active Ingredient Strength | 8 mg/1; mg/1 |
| Pharm Classes | Partial Opioid Agonists [MoA],Partial Opioid Agonist [EPC],Opioid Antagonist [EPC],Opioid Antagonists [MoA] |
| DEA Schedule | CIII |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 53217-328-01
30 POUCH in 1 CARTON (53217-328-01) > 1 FILM, SOLUBLE in 1 POUCH
| Marketing Start Date | 2010-09-13 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 53217-328-01 [53217032801]
Suboxone FILM, SOLUBLE
| Marketing Category | NDA |
| Application Number | NDA022410 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 2010-09-13 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| BUPRENORPHINE HYDROCHLORIDE | 8 mg/1 |
OpenFDA Data
| SPL SET ID: | 54c487ac-750b-462e-a4cb-dcab1dd43ba8 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Pharmacological Class
- Partial Opioid Agonists [MoA]
- Partial Opioid Agonist [EPC]
- Opioid Antagonist [EPC]
- Opioid Antagonists [MoA]
NDC Crossover Matching brand name "Suboxone" or generic name "Buprenorphine Hydrochloride, Naloxone Hydrochloride"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 12496-1202 | Suboxone | buprenorphine hydrochloride, naloxone hydrochloride |
| 12496-1204 | Suboxone | buprenorphine hydrochloride, naloxone hydrochloride |
| 12496-1212 | Suboxone | Suboxone |
| 12496-1208 | Suboxone | Suboxone |
| 53217-328 | Suboxone | Suboxone |
| 55700-147 | Suboxone | Suboxone |
| 63629-4028 | Suboxone | Suboxone |
| 0781-7216 | BUPRENORPHINE AND NALOXONE | buprenorphine hydrochloride, naloxone hydrochloride |
| 0781-7227 | BUPRENORPHINE AND NALOXONE | buprenorphine hydrochloride, naloxone hydrochloride |
| 0781-7238 | BUPRENORPHINE AND NALOXONE | buprenorphine hydrochloride, naloxone hydrochloride |
| 0781-7249 | BUPRENORPHINE AND NALOXONE | buprenorphine hydrochloride, naloxone hydrochloride |
Trademark Results [Suboxone]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SUBOXONE 78303449 2917297 Dead/Cancelled |
Reckitt Benckiser Healthcare (UK) Limited 2003-09-22 |
 SUBOXONE 77727995 3917631 Live/Registered |
INDIVIOR UK LIMITED 2009-05-04 |
 SUBOXONE 75226449 2169133 Live/Registered |
INDIVIOR UK LIMITED 1997-01-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.