NDC 58264-0020
D-20
Bos Taurus Thymus, Thyroid, Unspecified, Bos Taurus Pituitary Gland, Posterior, Bos Taurus Pancreas, Bos Taurus Adrenal Gland, And Bos Taurus Ovary
D-20 is a Sublingual Solution/ Drops in the Human Otc Drug category. It is labeled and distributed by Dna Labs, Inc.. The primary component is Bos Taurus Thymus; Thyroid, Unspecified; Bos Taurus Pituitary Gland, Posterior; Bos Taurus Pancreas; Bos Taurus Adrenal Gland; Bos Taurus Ovary.
| Product ID | 58264-0020_ca025dfb-e11b-499f-b79f-9611d8f4ba73 |
| NDC | 58264-0020 |
| Product Type | Human Otc Drug |
| Proprietary Name | D-20 |
| Generic Name | Bos Taurus Thymus, Thyroid, Unspecified, Bos Taurus Pituitary Gland, Posterior, Bos Taurus Pancreas, Bos Taurus Adrenal Gland, And Bos Taurus Ovary |
| Dosage Form | Solution/ Drops |
| Route of Administration | SUBLINGUAL |
| Marketing Start Date | 1990-01-01 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / UNAPPROVED HOMEOPATHIC |
| Labeler Name | DNA Labs, Inc. |
| Substance Name | BOS TAURUS THYMUS; THYROID, UNSPECIFIED; BOS TAURUS PITUITARY GLAND, POSTERIOR; BOS TAURUS PANCREAS; BOS TAURUS ADRENAL GLAND; BOS TAURUS OVARY |
| Active Ingredient Strength | 12 [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 58264-0020-1
29.57 mL in 1 BOTTLE, GLASS (58264-0020-1)
| Marketing Start Date | 1990-01-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 58264-0020-1 [58264002001]
D-20 SOLUTION/ DROPS
| Marketing Category | UNAPPROVED HOMEOPATHIC |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 1990-01-01 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| BOS TAURUS THYMUS | 12 [hp_X]/mL |
OpenFDA Data
| SPL SET ID: | d9699a09-5dd4-4d45-8069-edf48d681ce9 |
| Manufacturer | |
| UNII |
Trademark Results [D-20]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
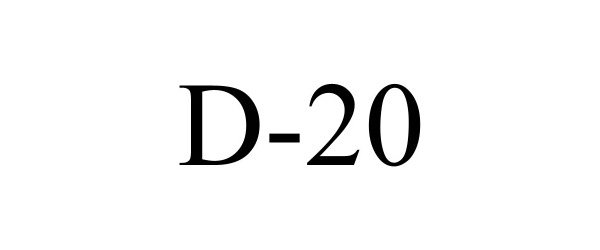 D-20 98614384 not registered Live/Pending |
Timestop Technologies, Inc. 2024-06-23 |
 D-20 73330685 1208680 Dead/Cancelled |
Victor United, Inc. 1981-10-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.