NDC 58264-0321
A-29
Cinnamon, Garlic, Ginger, Mustard Seed, Unspecified, And Nutmeg
A-29 is a Sublingual Solution in the Human Otc Drug category. It is labeled and distributed by Dna Labs, Inc.. The primary component is Cinnamon; Garlic; Ginger; Mustard Seed, Unspecified; Nutmeg.
| Product ID | 58264-0321_286c2ba3-d3ff-4665-806c-92e9b60545c0 |
| NDC | 58264-0321 |
| Product Type | Human Otc Drug |
| Proprietary Name | A-29 |
| Generic Name | Cinnamon, Garlic, Ginger, Mustard Seed, Unspecified, And Nutmeg |
| Dosage Form | Solution |
| Route of Administration | SUBLINGUAL |
| Marketing Start Date | 1990-01-01 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / UNAPPROVED HOMEOPATHIC |
| Labeler Name | DNA Labs, Inc. |
| Substance Name | CINNAMON; GARLIC; GINGER; MUSTARD SEED, UNSPECIFIED; NUTMEG |
| Active Ingredient Strength | 30 [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL; [hp_X]/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 58264-0321-1
29.57 mL in 1 BOTTLE, GLASS (58264-0321-1)
| Marketing Start Date | 1990-01-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 58264-0321-1 [58264032101]
A-29 SOLUTION
| Marketing Category | UNAPPROVED HOMEOPATHIC |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 1990-01-01 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| CINNAMON | 30 [hp_X]/mL |
OpenFDA Data
| SPL SET ID: | 0367b7d6-153b-4d9a-b8b9-4c29b2094a80 |
| Manufacturer | |
| UNII |
Trademark Results [A-29]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
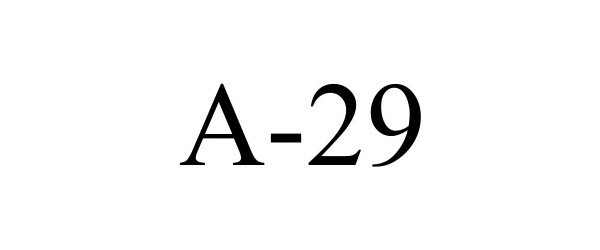 A-29 98110538 not registered Live/Pending |
EMBRAER S.A. 2023-07-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.