NDC 63941-023
Best Choice
Calcium Polycarbophil
Best Choice is a Oral Tablet, Film Coated in the Human Otc Drug category. It is labeled and distributed by Best Choice (valu Merchandisers Company). The primary component is Calcium Polycarbophil.
| Product ID | 63941-023_24eb538f-abcb-4a91-b691-a8aac01c952a |
| NDC | 63941-023 |
| Product Type | Human Otc Drug |
| Proprietary Name | Best Choice |
| Generic Name | Calcium Polycarbophil |
| Dosage Form | Tablet, Film Coated |
| Route of Administration | ORAL |
| Marketing Start Date | 2019-04-16 |
| Marketing Category | OTC MONOGRAPH NOT FINAL / OTC MONOGRAPH NOT FINAL |
| Application Number | part334 |
| Labeler Name | BEST CHOICE (VALU MERCHANDISERS COMPANY) |
| Substance Name | CALCIUM POLYCARBOPHIL |
| Active Ingredient Strength | 625 mg/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 63941-023-90
90 TABLET, FILM COATED in 1 BOTTLE (63941-023-90)
| Marketing Start Date | 2019-04-16 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 63941-023-90 [63941002390]
Best Choice TABLET, FILM COATED
| Marketing Category | OTC monograph not final |
| Application Number | part334 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2019-04-16 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| CALCIUM POLYCARBOPHIL | 625 mg/1 |
OpenFDA Data
| SPL SET ID: | 24eb538f-abcb-4a91-b691-a8aac01c952a |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI | |
| UPC Code |
NDC Crossover Matching brand name "Best Choice" or generic name "Calcium Polycarbophil"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 63941-720 | Best Choice | Best Choice |
| 63941-023 | Best Choice | Best Choice |
| 63941-709 | Best Choice | Best Choice |
| 63941-716 | Best Choice | Best Choice |
| 21130-021 | Fiber Caplets | Calcium Polycarbophil |
| 30142-132 | Fiber Caplets | Calcium Polycarbophil |
| 0536-4306 | Fiber Lax | Calcium Polycarbophil |
| 0615-5532 | Fiber Lax | CALCIUM POLYCARBOPHIL |
| 21130-147 | Fiber Laxative | CALCIUM POLYCARBOPHIL |
| 30142-477 | fiber laxative | Calcium polycarbophil |
| 36800-465 | Fiber Laxative | Calcium polycarbophil |
| 0904-2500 | FIBER TABS | calcium polycarbophil |
| 0005-2500 | FIBERCON | calcium polycarbophil |
| 24385-125 | Good Neighbor Pharmacy Fiber-Caps Fiber Laxative | Calcium Polycarbophil |
| 0113-0477 | Good Sense Fiber Laxative | Calcium polycarbophil |
| 0224-0500 | KONSYL Daily Therapy Fiber | CALCIUM POLYCARBOPHIL |
| 0224-1889 | Konsyl Fiber | CALCIUM POLYCARBOPHIL |
| 37205-213 | Leader Fiber | Calcium polycarbophil |
| 11822-2025 | Rite Aid Fiber Caplets | Calcium Polycarbophil |
| 36800-477 | Topcare Fiber Laxative | Calcium polycarbophil |
| 11673-620 | up and up fiber therapy | Calcium polycarbophil |
| 0363-0120 | Walgreen Fiber Lax | Calcium Polycarbophil |
Trademark Results [Best Choice]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BEST CHOICE 98865849 not registered Live/Pending |
Associated Wholesale Grocers, Inc. 2024-11-21 |
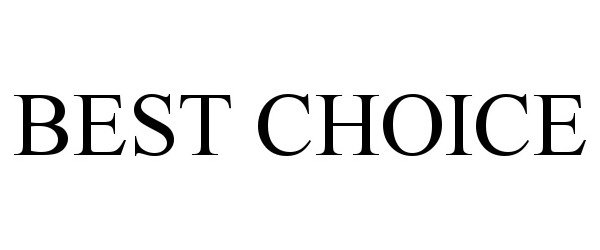 BEST CHOICE 97120427 not registered Live/Pending |
Associated Wholesale Grocers, Inc. 2021-11-11 |
 BEST CHOICE 87312368 not registered Dead/Abandoned |
MICHAEL SHEK 2017-01-24 |
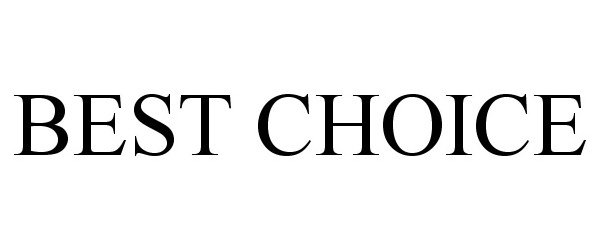 BEST CHOICE 86817655 5152258 Live/Registered |
Space Flex International LLC 2015-11-12 |
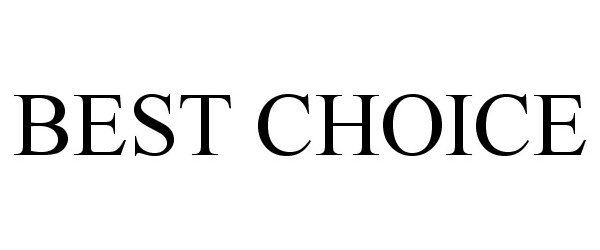 BEST CHOICE 86656373 not registered Dead/Abandoned |
Grocery Exports, Inc. 2015-06-09 |
 BEST CHOICE 85060923 not registered Dead/Abandoned |
ClearChoice Holdings LLC 2010-06-11 |
 BEST CHOICE 79110183 not registered Dead/Abandoned |
SPECIAL FRUIT NV 2012-01-11 |
 BEST CHOICE 77975712 3493774 Dead/Cancelled |
Associated Wholesale Grocers, Inc. 2007-04-12 |
 BEST CHOICE 77154903 3499694 Dead/Cancelled |
Associated Wholesale Grocers, Inc. 2007-04-12 |
 BEST CHOICE 77154886 3838242 Dead/Cancelled |
Associated Wholesale Grocers, Inc. 2007-04-12 |
 BEST CHOICE 77154870 3524931 Dead/Cancelled |
Associated Wholesale Grocers, Inc. 2007-04-12 |
 BEST CHOICE 77154857 3524930 Dead/Cancelled |
Associated Wholesale Grocers, Inc. 2007-04-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.