NDC 65785-169
PUR-WASH
Water
PUR-WASH is a Ophthalmic Solution in the Human Otc Drug category. It is labeled and distributed by Niagara Pharmaceuticals Inc.. The primary component is Water.
| Product ID | 65785-169_bed9635f-79f8-9503-e053-2a95a90a013e |
| NDC | 65785-169 |
| Product Type | Human Otc Drug |
| Proprietary Name | PUR-WASH |
| Generic Name | Water |
| Dosage Form | Solution |
| Route of Administration | OPHTHALMIC |
| Marketing Start Date | 2011-09-12 |
| Marketing Category | NDA / |
| Application Number | NDA022305 |
| Labeler Name | Niagara Pharmaceuticals Inc. |
| Substance Name | WATER |
| Active Ingredient Strength | 929 g/946mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 65785-169-01
30 mL in 1 BOTTLE, UNIT-DOSE (65785-169-01)
| Marketing Start Date | 2011-09-12 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "PUR-WASH" or generic name "Water"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 65785-162 | PUR-WASH | PUR-WASH |
| 65785-164 | PUR-WASH | PUR-WASH |
| 65785-168 | Pur-Wash | Pur-Wash |
| 65785-166 | PUR-WASH | PUR-WASH |
| 65785-160 | PUR-WASH | PUR-WASH |
| 0404-9970 | Bacteriostatic Water | Water |
| 0173-0518 | Diluent | water |
| 0173-0857 | Diluent | water |
| 0404-7198 | Eyewash | Water |
| 0264-2101 | Sterile Water | Water |
| 0264-7385 | Sterile Water | Water |
| 0264-7850 | Sterile Water | Water |
| 0338-0003 | Sterile Water | Water |
| 0338-0004 | Sterile Water | Water |
| 0338-0013 | Sterile Water | Water |
| 0404-9959 | Sterile Water | Water |
| 0404-9971 | STERILE WATER | WATER |
| 0409-4887 | Sterile Water | WATER |
Trademark Results [PUR-WASH]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
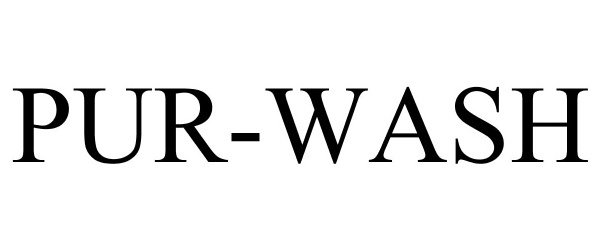 PUR-WASH 88122071 not registered Live/Pending |
Niagara Pharmaceuticals Inc. 2018-09-18 |
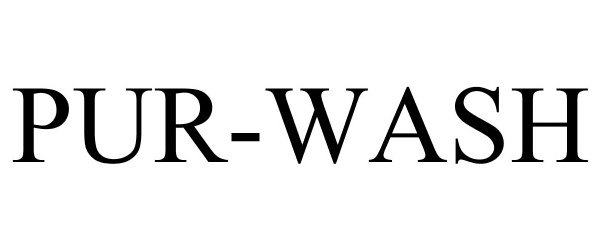 PUR-WASH 85433300 4401858 Live/Registered |
Niagara Pharmaceuticals Inc. 2011-09-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.