NDC 66220-422
Omeclamox-Pak
Omeprazole, Clarithromycin, Amoxicillin
Omeclamox-Pak is a Kit in the Human Prescription Drug category. It is labeled and distributed by Cumberland Pharmaceuticals Inc.. The primary component is .
| Product ID | 66220-422_13c419e8-64ba-41d9-ae34-17ce8596bcd7 |
| NDC | 66220-422 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Omeclamox-Pak |
| Generic Name | Omeprazole, Clarithromycin, Amoxicillin |
| Dosage Form | Kit |
| Marketing Start Date | 2012-04-27 |
| Marketing Category | NDA / NDA |
| Application Number | NDA050824 |
| Labeler Name | Cumberland Pharmaceuticals Inc. |
| Active Ingredient Strength | 0 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 66220-422-01
1 KIT in 1 BLISTER PACK (66220-422-01)
| Marketing Start Date | 2012-04-27 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 66220-422-01 [66220042201]
Omeclamox-Pak KIT
| Marketing Category | NDA |
| Application Number | NDA050824 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2012-04-27 |
NDC 66220-422-02 [66220042202]
Omeclamox-Pak KIT
| Marketing Category | NDA |
| Application Number | NDA050824 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2012-04-27 |
NDC 66220-422-11 [66220042211]
Omeclamox-Pak KIT
| Marketing Category | NDA |
| Application Number | NDA050824 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 2012-04-27 |
| Marketing End Date | 2016-07-25 |
Drug Details
OpenFDA Data
| SPL SET ID: | 9e52c29a-fdbe-4a4d-b280-d700eba5b94f |
| Manufacturer | |
| RxNorm Concept Unique ID - RxCUI |
Trademark Results [Omeclamox-Pak]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
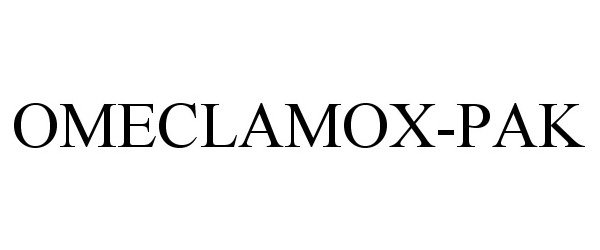 OMECLAMOX-PAK 86170802 4588520 Live/Registered |
CUMBERLAND PHARMACEUTICALS INC. 2014-01-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.