NDC 66302-732
Tyvaso DPI
Treprostinil
Tyvaso DPI is a Oral Inhalant in the Human Prescription Drug category. It is labeled and distributed by United Therapeutics Corporation. The primary component is Treprostinil.
| Product ID | 66302-732_46c30322-3a4d-41f0-b7c1-1e4aba4831be |
| NDC | 66302-732 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Tyvaso DPI |
| Generic Name | Treprostinil |
| Dosage Form | Inhalant |
| Route of Administration | ORAL |
| Marketing Start Date | 2022-05-23 |
| Marketing Category | NDA / |
| Application Number | NDA214324 |
| Labeler Name | United Therapeutics Corporation |
| Substance Name | TREPROSTINIL |
| Active Ingredient Strength | 32 ug/1 |
| Pharm Classes | Prostacycline Vasodilator [EPC], Prostaglandins I [CS], Vasodilation [PE] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 66302-732-04
1 BLISTER PACK in 1 KIT (66302-732-04) > 4 BLISTER PACK in 1 BLISTER PACK > 4 INHALANT in 1 BLISTER PACK
| Marketing Start Date | 2022-05-23 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Tyvaso DPI" or generic name "Treprostinil"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 66302-101 | Remodulin | treprostinil |
| 66302-102 | Remodulin | treprostinil |
| 0703-0666 | Treprostinil | Treprostinil |
| 0703-0676 | Treprostinil | Treprostinil |
| 0703-0686 | Treprostinil | Treprostinil |
| 0703-0696 | Treprostinil | Treprostinil |
| 0781-3420 | Treprostinil | treprostinil |
| 0781-3425 | Treprostinil | treprostinil |
| 0781-3427 | Treprostinil | treprostinil |
| 0781-3430 | Treprostinil | treprostinil |
| 42023-206 | Treprostinil | Treprostinil |
| 42023-207 | Treprostinil | Treprostinil |
| 42023-208 | Treprostinil | Treprostinil |
| 42023-209 | Treprostinil | Treprostinil |
| 62332-514 | Treprostinil | Treprostinil |
| 62332-515 | Treprostinil | Treprostinil |
| 62332-516 | Treprostinil | Treprostinil |
| 62332-517 | Treprostinil | Treprostinil |
Trademark Results [Tyvaso DPI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
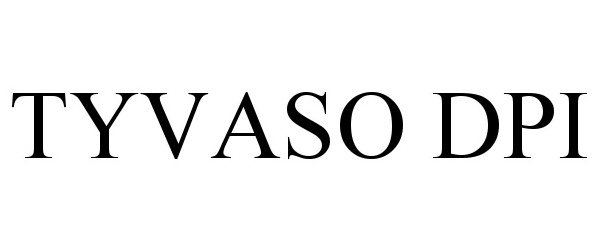 TYVASO DPI 90370386 not registered Live/Pending |
United Therapeutics Corporation 2020-12-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.