NDC 66312-441
Polocaine
Mepivacaine Hydrochloride
Polocaine is a Subcutaneous Injection, Solution in the Human Prescription Drug category. It is labeled and distributed by Dentsply Pharmaceutical. The primary component is Mepivacaine Hydrochloride.
| Product ID | 66312-441_1dd09e2e-67de-4b76-a024-ffd02b62ea00 |
| NDC | 66312-441 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Polocaine |
| Generic Name | Mepivacaine Hydrochloride |
| Dosage Form | Injection, Solution |
| Route of Administration | SUBCUTANEOUS |
| Marketing Start Date | 1984-10-10 |
| Marketing Category | ANDA / ANDA |
| Application Number | ANDA088387 |
| Labeler Name | Dentsply Pharmaceutical |
| Substance Name | MEPIVACAINE HYDROCHLORIDE |
| Active Ingredient Strength | 30 mg/mL |
| Pharm Classes | Amide Local Anesthetic [EPC],Amides [CS],Local Anesthesia [PE] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 66312-441-16
50 CARTRIDGE in 1 CARTON (66312-441-16) > 1.7 mL in 1 CARTRIDGE
| Marketing Start Date | 1984-10-10 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 66312-441-16 [66312044116]
Polocaine INJECTION, SOLUTION
| Marketing Category | ANDA |
| Application Number | ANDA088387 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | ML |
| Marketing Start Date | 1984-10-10 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| MEPIVACAINE HYDROCHLORIDE | 30 mg/mL |
OpenFDA Data
| SPL SET ID: | 25074a6a-7f67-4ad8-86e0-2afe23ec5d04 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Pharmacological Class
- Amide Local Anesthetic [EPC]
- Amides [CS]
- Local Anesthesia [PE]
NDC Crossover Matching brand name "Polocaine" or generic name "Mepivacaine Hydrochloride"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 50090-4408 | Polocaine | MEPIVACAINE HYDROCHLORIDE |
| 50090-4517 | Polocaine | MEPIVACAINE HYDROCHLORIDE |
| 63323-283 | Polocaine | Polocaine |
| 63323-296 | Polocaine | Polocaine |
| 66312-441 | Polocaine | Polocaine |
| 0362-0753 | Carbocaine | Mepivacaine Hydrochloride |
| 0409-1036 | Carbocaine | Mepivacaine Hydrochloride |
| 0409-1038 | Carbocaine | Mepivacaine Hydrochloride |
| 0409-1041 | Carbocaine | Mepivacaine Hydrochloride |
| 0409-1067 | Carbocaine | Mepivacaine Hydrochloride |
| 0409-2047 | Carbocaine | Mepivacaine Hydrochloride |
| 31382-753 | Carbocaine | Mepivacaine hydrochloride |
| 42756-1080 | IQ Dental Mepivacaine | Mepivacaine Hydrochloride |
| 43128-005 | Mepivacaine | Mepivacaine Hydrochloride |
| 50227-1080 | Mepivacaine | Mepivacaine Hydrochloride |
| 0404-6730 | Mepivacaine Hydrochloride | Mepivacaine Hydrochloride |
| 43305-1080 | Pearson Mepivacaine | Mepivacaine Hydrochloride |
| 0362-1098 | Scandonest 3% Plain | Mepivacaine Hydrochloride |
Trademark Results [Polocaine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
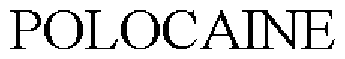 POLOCAINE 73499932 1376828 Live/Registered |
ASTRA PHARMACEUTICAL PRODUCTS, INC. 1984-09-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.