NDC 66490-690
Mysoline
Primidone
Mysoline is a Oral Tablet in the Human Prescription Drug category. It is labeled and distributed by Valeant Pharmaceuticals North America. The primary component is Primidone.
| Product ID | 66490-690_16ea26a8-99f6-43bb-bff4-588e95d9d508 |
| NDC | 66490-690 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Mysoline |
| Generic Name | Primidone |
| Dosage Form | Tablet |
| Route of Administration | ORAL |
| Marketing Start Date | 2009-06-24 |
| Marketing Category | NDA / NDA |
| Application Number | NDA009170 |
| Labeler Name | Valeant Pharmaceuticals North America |
| Substance Name | PRIMIDONE |
| Active Ingredient Strength | 50 mg/1 |
| Pharm Classes | Anti-epileptic Agent [EPC],Decreased Central Nervous System Disorganized Electrical Activity [PE] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 66490-690-10
100 TABLET in 1 BOTTLE (66490-690-10)
| Marketing Start Date | 2009-06-24 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 66490-690-50 [66490069050]
Mysoline TABLET
| Marketing Category | NDA |
| Application Number | NDA009170 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 2009-06-24 |
| Marketing End Date | 2010-06-07 |
NDC 66490-690-10 [66490069010]
Mysoline TABLET
| Marketing Category | NDA |
| Application Number | NDA009170 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2009-06-24 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| PRIMIDONE | 50 mg/1 |
OpenFDA Data
| SPL SET ID: | af593171-dabb-4ea3-b44c-89ed457b2c46 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Pharmacological Class
- Anti-epileptic Agent [EPC]
- Decreased Central Nervous System Disorganized Electrical Activity [PE]
Medicade Reported Pricing
66490069010 MYSOLINE 50 MG TABLET
Pricing Unit: EA | Drug Type:
NDC Crossover Matching brand name "Mysoline" or generic name "Primidone"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 66490-691 | Mysoline | Mysoline |
| 66490-690 | Mysoline | Mysoline |
| 0143-1482 | Primidone | Primidone |
| 0143-1484 | Primidone | Primidone |
| 0527-1231 | Primidone | Primidone |
| 0527-1301 | Primidone | Primidone |
| 0591-5321 | Primidone | Primidone |
| 0603-5371 | Primidone | primidone |
| 0603-5372 | Primidone | primidone |
| 0615-2521 | Primidone | primidone |
| 0615-5591 | Primidone | Primidone |
| 0615-7738 | Primidone | Primidone |
| 0615-7936 | Primidone | primidone |
| 0615-8206 | Primidone | Primidone |
| 0904-5559 | Primidone | Primidone |
| 10135-522 | Primidone | Primidone |
| 10135-540 | Primidone | Primidone |
| 42291-508 | Primidone | primidone |
| 42291-509 | Primidone | Primidone |
| 42291-511 | Primidone | Primidone |
| 50090-1622 | Primidone | Primidone |
| 50090-1636 | Primidone | Primidone |
| 50090-4083 | Primidone | Primidone |
| 50268-686 | Primidone | Primidone |
| 50268-687 | Primidone | Primidone |
| 51407-637 | Primidone | Primidone |
| 51407-638 | Primidone | Primidone |
| 53746-544 | Primidone | Primidone |
Trademark Results [Mysoline]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
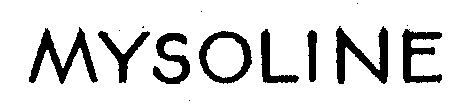 MYSOLINE 71620216 0569143 Live/Registered |
IMPERIAL CHEMICAL (PHARMACEUTICALS) LIMITED 1951-10-19 |
© 2025 FDA.report
This site is not affiliated with or endorsed by the FDA.