NDC 67457-177
RIMSO-50
Dimethyl Sulfoxide
RIMSO-50 is a Intravesical Irrigant in the Human Prescription Drug category. It is labeled and distributed by Mylan Institutional Llc. The primary component is Dimethyl Sulfoxide.
| Product ID | 67457-177_5887674e-24ed-4e16-a4c4-6c25350bae3e |
| NDC | 67457-177 |
| Product Type | Human Prescription Drug |
| Proprietary Name | RIMSO-50 |
| Generic Name | Dimethyl Sulfoxide |
| Dosage Form | Irrigant |
| Route of Administration | INTRAVESICAL |
| Marketing Start Date | 1978-04-04 |
| Marketing Category | NDA / NDA |
| Application Number | NDA017788 |
| Labeler Name | Mylan Institutional LLC |
| Substance Name | DIMETHYL SULFOXIDE |
| Active Ingredient Strength | 1 g/mL |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 67457-177-50
1 VIAL, GLASS in 1 CARTON (67457-177-50) > 50 mL in 1 VIAL, GLASS
| Marketing Start Date | 1978-04-04 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 67457-177-50 [67457017750]
RIMSO-50 IRRIGANT
| Marketing Category | NDA |
| Application Number | NDA017788 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | ML |
| Marketing Start Date | 1978-04-04 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| DIMETHYL SULFOXIDE | .54 g/mL |
OpenFDA Data
| SPL SET ID: | 58b25d79-78f2-4953-b0c6-61658dc4ef0d |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Medicade Reported Pricing
67457017750 RIMSO-50 SOLUTION
Pricing Unit: ML | Drug Type:
Trademark Results [RIMSO-50]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
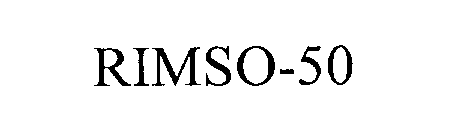 RIMSO-50 76267004 2529846 Live/Registered |
MYLAN TEORANTA 2001-06-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.