NDC 67457-845
OGIVRI
Trastuzumab
OGIVRI is a Intravenous Injection, Powder, Lyophilized, For Solution in the Human Prescription Drug category. It is labeled and distributed by Mylan Institutional Llc. The primary component is Trastuzumab.
| Product ID | 67457-845_476fea90-ea21-4542-80eb-e3cf13653ad9 |
| NDC | 67457-845 |
| Product Type | Human Prescription Drug |
| Proprietary Name | OGIVRI |
| Generic Name | Trastuzumab |
| Dosage Form | Injection, Powder, Lyophilized, For Solution |
| Route of Administration | INTRAVENOUS |
| Marketing Start Date | 2019-11-29 |
| Marketing Category | BLA / BLA |
| Application Number | BLA761074 |
| Labeler Name | Mylan Institutional LLC |
| Substance Name | TRASTUZUMAB |
| Active Ingredient Strength | 420 mg/20mL |
| Pharm Classes | HER2/neu Receptor Antagonist [EPC],HER2/Neu/cerbB2 Antagonists [MoA] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Drug Details
NDC Crossover Matching brand name "OGIVRI" or generic name "Trastuzumab"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 67457-845 | OGIVRI | trastuzumab |
| 67457-847 | OGIVRI | trastuzumab |
| 67457-991 | OGIVRI | trastuzumab |
| 50242-132 | Herceptin | Trastuzumab |
| 50242-134 | Herceptin | Trastuzumab |
| 50242-333 | Herceptin | Trastuzumab |
| 63459-303 | HERZUMA | TRASTUZUMAB |
| 63459-305 | HERZUMA | TRASTUZUMAB |
| 0006-5033 | Ontruzant | trastuzumab |
| 0069-0305 | Trazimera-qyyp | trastuzumab |
Trademark Results [OGIVRI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
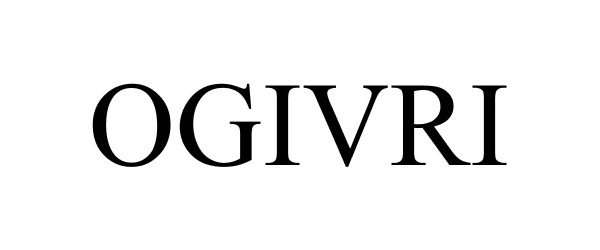 OGIVRI 88650753 not registered Live/Pending |
Mylan Institutional Inc. 2019-10-11 |
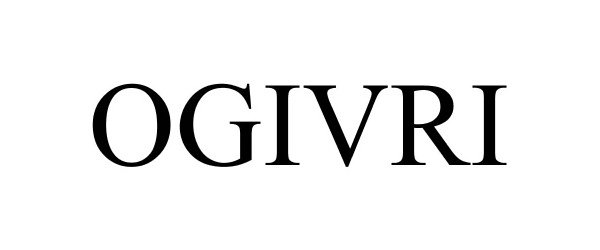 OGIVRI 86970805 not registered Live/Pending |
Mylan Institutional Inc. 2016-04-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.