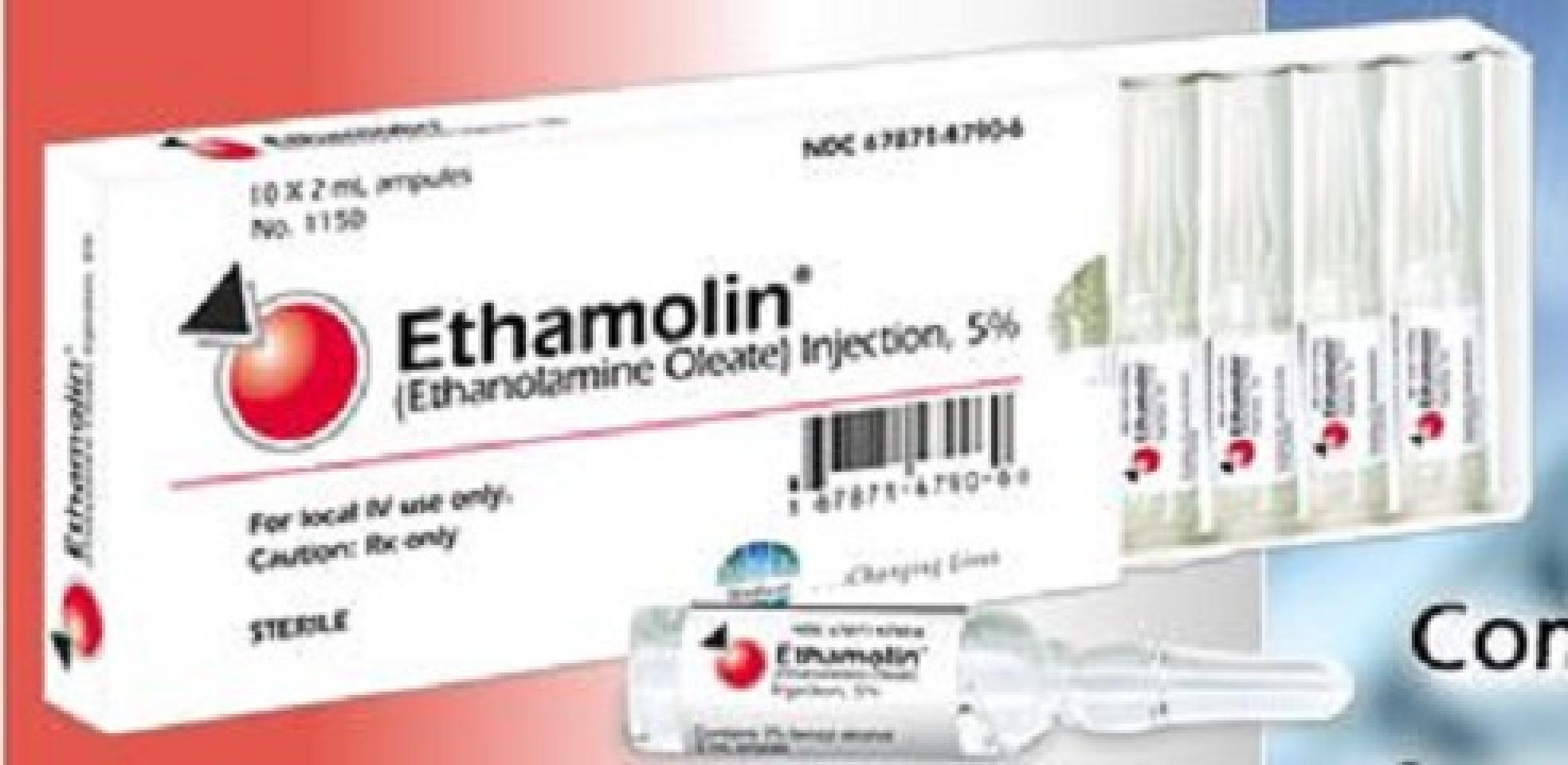
NDC 67871-479
Ethamolin
Ethanolamine Oleate
Ethamolin is a Intravenous Injection, Solution in the Human Prescription Drug category. It is labeled and distributed by Qol Medical, Llc.. The primary component is Ethanolamine Oleate.
| Product ID | 67871-479_179b54fc-984c-427c-899f-b940bc1512c8 |
| NDC | 67871-479 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Ethamolin |
| Generic Name | Ethanolamine Oleate |
| Dosage Form | Injection, Solution |
| Route of Administration | INTRAVENOUS |
| Marketing Start Date | 1998-12-22 |
| Marketing Category | NDA / NDA |
| Application Number | NDA019357 |
| Labeler Name | QOL Medical, LLC. |
| Substance Name | ETHANOLAMINE OLEATE |
| Active Ingredient Strength | 50 mg/mL |
| Pharm Classes | Sclerosing Activity [MoA],Sclerosing Agent [EPC],Vascular Sclerosing Activity [PE] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 67871-479-06
10 AMPULE in 1 BOX (67871-479-06) > 2 mL in 1 AMPULE
| Marketing Start Date | 1998-12-22 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 67871-479-06 [67871047906]
Ethamolin INJECTION, SOLUTION
| Marketing Category | NDA |
| Application Number | NDA019357 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 1998-12-22 |
| Inactivation Date | 2020-01-31 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| ETHANOLAMINE OLEATE | 50 mg/mL |
OpenFDA Data
| SPL SET ID: | a9c1d8c5-443e-400e-a42c-16a25fb47231 |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Pharmacological Class
- Sclerosing Activity [MoA]
- Sclerosing Agent [EPC]
- Vascular Sclerosing Activity [PE]
Trademark Results [Ethamolin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ETHAMOLIN 73520081 1374539 Live/Registered |
GLAXO GROUP LIMITED 1985-01-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.