NDC 69578-268
Hexagen
Skin And Nasal Antiseptic
Hexagen is a Nasal; Topical Liquid in the Human Otc Drug category. It is labeled and distributed by Global Health Solutions Dba Turn Therapeutics. The primary component is Benzalkonium Chloride.
| Product ID | 69578-268_aa6eef2d-d753-2117-e053-2995a90aff4f |
| NDC | 69578-268 |
| Product Type | Human Otc Drug |
| Proprietary Name | Hexagen |
| Generic Name | Skin And Nasal Antiseptic |
| Dosage Form | Liquid |
| Route of Administration | NASAL; TOPICAL |
| Marketing Start Date | 2020-07-14 |
| Marketing Category | OTC MONOGRAPH NOT FINAL / OTC MONOGRAPH NOT FINAL |
| Application Number | part333E |
| Labeler Name | Global Health Solutions DBA Turn Therapeutics |
| Substance Name | BENZALKONIUM CHLORIDE |
| Active Ingredient Strength | 0 g/100g |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 69578-268-30
30 g in 1 CARTON (69578-268-30)
| Marketing Start Date | 2020-07-14 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
Trademark Results [Hexagen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
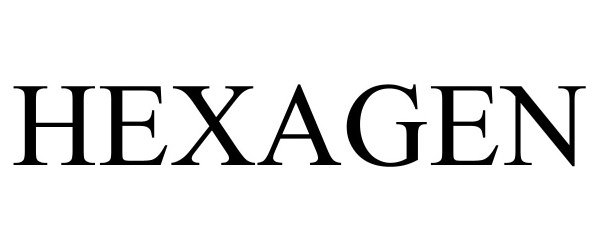 HEXAGEN 97880804 not registered Live/Pending |
Affera, Inc 2023-04-10 |
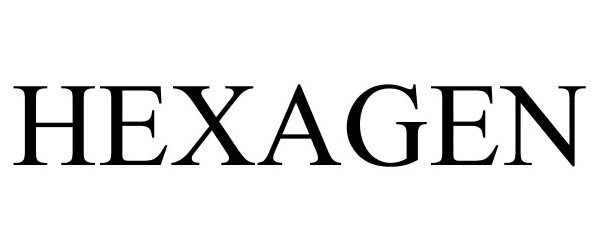 HEXAGEN 88238897 not registered Live/Pending |
MORPHOGEN NUTRITION LLC 2018-12-21 |
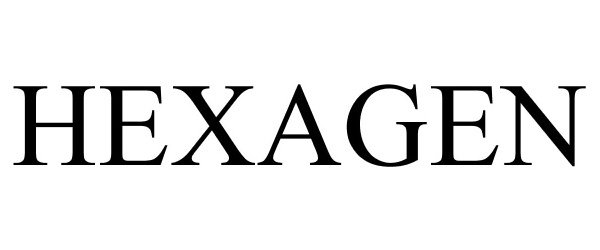 HEXAGEN 87334512 5285038 Live/Registered |
Bradley E. Burnam 2017-02-14 |
 HEXAGEN 79386704 not registered Live/Pending |
Libertine FPE Limited 2023-07-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.