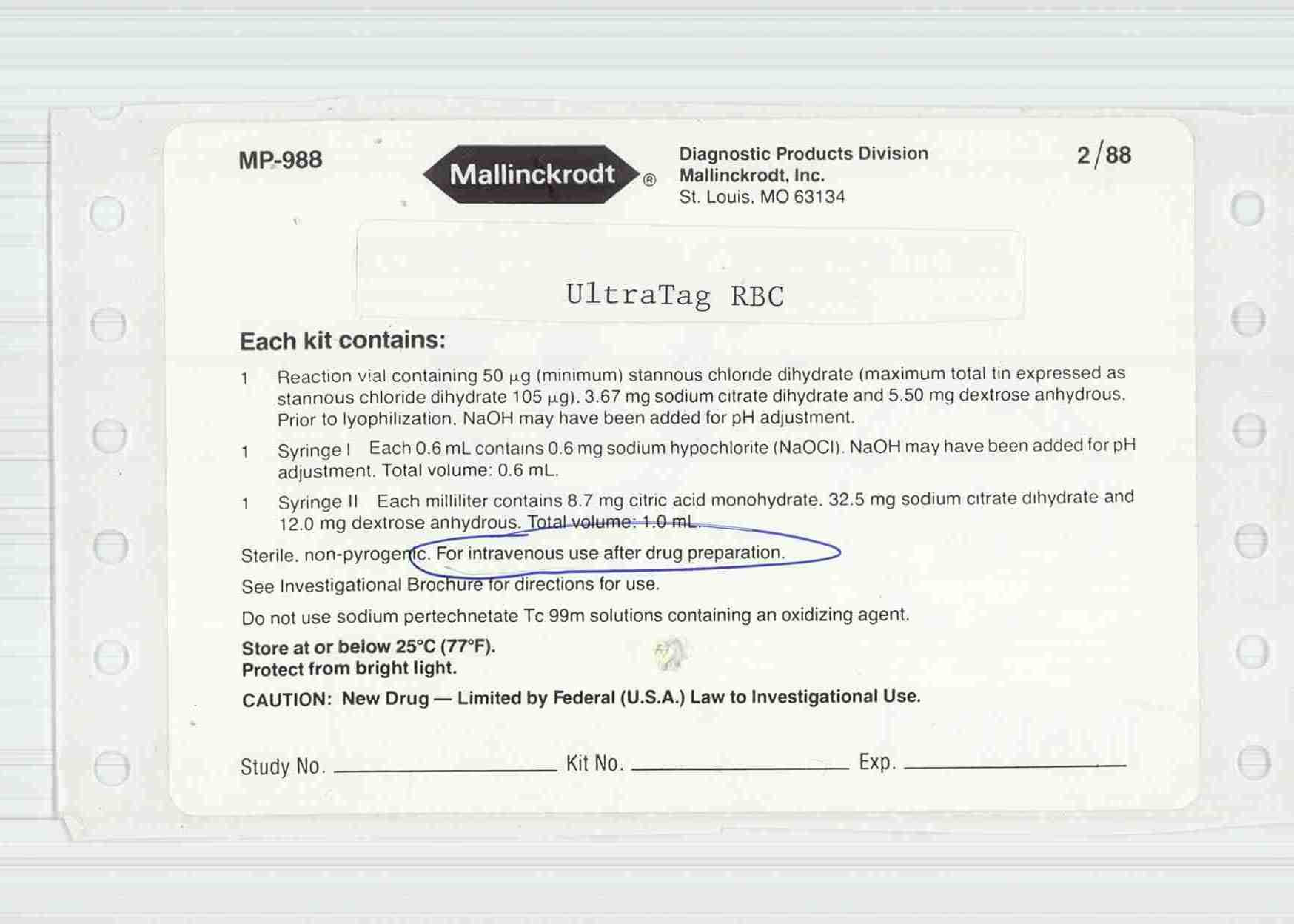
NDC 69945-068
Ultratag RBC
Technetium Tc 99m-labeled Red Blood Cells
Ultratag RBC is a Kit in the Human Prescription Drug category. It is labeled and distributed by Curium Us Llc. The primary component is .
| Product ID | 69945-068_0dc8f021-eb47-48e6-9682-8f12ef25f330 |
| NDC | 69945-068 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Ultratag RBC |
| Generic Name | Technetium Tc 99m-labeled Red Blood Cells |
| Dosage Form | Kit |
| Marketing Start Date | 1991-06-10 |
| Marketing Category | NDA / NDA |
| Application Number | NDA019981 |
| Labeler Name | Curium US LLC |
| Active Ingredient Strength | 0 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 69945-068-20
5 CELLO PACK in 1 BOX (69945-068-20) > 1 KIT in 1 CELLO PACK * .6 mL in 1 SYRINGE, GLASS * 1 mL in 1 SYRINGE, GLASS * 9.5 mg in 1 VIAL, GLASS
| Marketing Start Date | 1991-06-10 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 69945-068-20 [69945006820]
Ultratag RBC KIT
| Marketing Category | NDA |
| Application Number | NDA019981 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Marketing Start Date | 1991-06-10 |
Drug Details
OpenFDA Data
| SPL SET ID: | 9f999b75-8a66-40bf-9352-478850b41225 |
| Manufacturer |
Trademark Results [Ultratag]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ULTRATAG 75429998 2330766 Live/Registered |
Sentech EAS Corporation 1998-02-06 |
 ULTRATAG 75146549 2137927 Dead/Cancelled |
RANDTEC, INCORPORATED 1996-08-07 |
 ULTRATAG 73811821 1618372 Live/Registered |
MALLINCKRODT, INC. 1989-07-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.